June 2, 2020 | Kathryn Tworkoski, PhD, RAC | Principal Clinical Research Scientist
In the past, we’ve written blog posts explaining what an Investigational New Drug (IND) is, providing guidance on pre-IND meetings, and outlining the submission and maintenance of INDs. In these earlier posts, we mentioned that INDs are “living” documents, which means that they are continuously being updated as a drug development program proceeds. So today, we thought we’d take a few minutes to discuss the types of IND updates that you may come across. Let’s start with…
Reasons to update your IND
Broadly speaking, the reasons to update an IND can be subdivided into a few main categories:
- Protocol Amendments: Adding a new protocol/investigator, or changing an existing protocol
- Information Amendments: Adding new data from nonclinical or clinical studies, or new Chemistry Manufacturing, and Controls (CMC) data
- Safety Reports: Information about any serious adverse events that occur during a clinical study that are both unexpected and considered related to study drug
- Response to feedback from the Food and Drug Administration (FDA) or request for FDA input: this is pretty self-explanatory!
- Annual updates: An FDA-mandated, annual report on activity conducted under an IND
Protocol amendments, safety reports, and responses to requests for FDA feedback are all generally filed in something like real-time. In some instances, there may be specific timelines that should be observed (eg, for safety reporting, or for certain types of FDA interactions). For the purposes of this overview, we’re not going to go into that level of detail (you can always reach out with any questions you may have!).
The timing of information amendments, on the other hand, can be a little more subjective. Generally, any new data that are considered especially important to a drug development program (such as novel safety findings or changes to drug manufacturing processes) should be added to an IND as soon as they become available. However, a large amount of the data generated during drug development may not have an immediate impact on drug use or patient safety, and therefore does not need to be submitted to the IND right away. Instead, these data may be submitted as part of a regularly-scheduled, annual IND update.
What’s that? You want to know what’s included in an annual update and when it should be filed? Well, since you asked so nicely…
Annual IND updates
All Sponsors are required to provide an annual IND update and this update needs to be submitted to the FDA within 60 days of the anniversary that the IND cleared. (Remember that the FDA has 30 days to review an IND after it is submitted, and if there are no objections to the program, the IND is cleared at the end of the 30-day period.) In this way, the reporting period for IND updates starts on the day that the IND cleared and then recurs annually on that date.
Not surprisingly, the purpose of an IND update is to tell the FDA about all new data that have been obtained in the past year, including nonclinical data, clinical data, and CMC data. Basically, an IND update should contain:
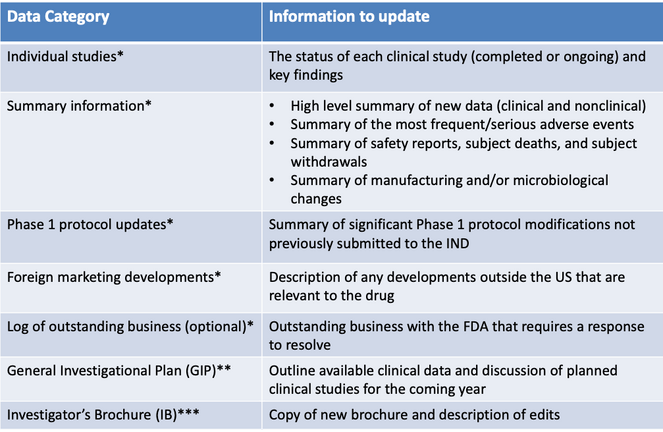
* These data categories may be presented in individual documents (annual report) or combined into a single Development Safety Update Report document (see below).
** The GIP may be a stand-alone document or may be combined with other data categories (see below).
*** The IB should always be presented as a separate, stand-alone document.
Of course, the data that you include will be dependent of your specific program. Generally speaking, an IND update should focus on events that occurred in the previous year. However, some aspects of an update may be cumulative (summaries of serious adverse events, for instance) and will therefore include data from outside the reporting period.
If any nonclinical or clinical studies have been completed during the past year, the finalized study report can be submitted with the annual IND update (assuming they haven’t already been submitted). Of course, it is very likely that studies will be ongoing at the time of the annual update. When this happens, nonclinical studies are generally described as “ongoing,” without the addition of preliminary/nonvalidated data. In contrast, a clinical data cut is taken at the end of the reporting period (on the anniversary of the date that the IND cleared), and the appropriate resulting data (typically safety data) are included in the annual IND update.
Annual reports vs DSURs
Ok, we’ve established the type of information that needs to be submitted to the FDA as part of an annual IND update. But what about the format of the update itself: how should it be presented?
There are two basic ways to submit an IND annual update: via an inventively titled “annual report,” or as a “Development Safety Update Report” (DSUR). An annual report is the more traditional way to update an IND, and it is specific to the US. An annual report is comprised of a series of small documents, each of which describes changes to a specific category of data (see the table above). The exact requirements for an IND annual report are outlined in the Code of Federal Regulations (CFR) (specifically 21 CFR 312.33, for those of you who are interested).

Image courtesy of Nutdanai Apikhomboonwaroot at FreeDigitalPhotos.net
In contrast, a DSUR is a single document that was originally developed in Europe and is accepted by both the FDA and the European Medicines Agency (EMA). Since DSURs are accepted by more agencies globally, they are becoming the more standard way to provide IND annual updates, and the International Council for Harmonisation E2F Guidance (Development Safety Update Report) provides an overview of the FDA’s thoughts on how a DSUR should be written and used.
Generally, the contents of a DSUR and annual report are fairly similar (as outlined above). However, the DSUR tends to be a little more detailed in its data analysis and has a slightly stronger focus on potential impacts on patients. For instance, DSURs have separate sections for actions taken for safety reasons, line listings for serious adverse reactions, and evaluations of potential benefits and risks for patients. Such stand-alone sections do not exist in the annual report format.
Regardless of whether an annual report or a DSUR is filed, the annual IND update should include an updated GIP and an updated IB (if the IB was updated during the reporting period). An IB is submitted as a stand-alone document, regardless of whether you file an annual report or a DSUR. On the other hand, a GIP is a stand-alone document if you file an annual report. But if you file a DSUR, the General Investigational Plan may be a stand‑alone document or may be added to the DSUR appendices—either way is fine.
What if I have no new data to report?
This actually isn’t as uncommon as you might think! Development programs may be delayed or placed on hold for a number of reasons, including unexpected data, manufacturing adjustments, financial concerns, or shifting business priorities.
Regardless of the amount of new data generated in a given year, the FDA expects to see an annual IND update (as either an annual report or a DSUR) as long as the IND remains active. This just means that if there are no new data to report in a given year, the amount of work that goes into preparing an annual report/DSUR is greatly reduced. For an annual report, sections related to information or data for which there are no updates can be excluded. For a DSUR, a statement that no new data are available and/or repetition of previously submitted key data (eg, cumulative summaries of exposure or serious adverse events) is sufficient. In either case, a description of updated clinical plans for the coming year (if any) should also be provided in the GIP.

Image courtesy of David Castillo Dominici at FreeDigitalPhotos.net
What if I have more than 1 IND for a drug?
You may have multiple open INDs for the same drug if, for example, you are studying a drug in several indications. So do you have to file a separate annual update for each IND? Well, the answer is both yes and no…
You do have to provide an annual update for each IND. However, each IND update can contain the same information: that way you only have to write up one series of documents, and those same documents can be submitted to all INDs. This has the benefit of saving the Sponsor a bit of time/effort, and also creates a common repository for all data related to a given drug.
Of course, if you want to submit the same annual update for multiple INDs, you need to submit them at the same time. (And if you are studying your drug in multiple countries, you may also want to submit required annual updates to foreign regulatory authorities at the same time.) To do that, you need to harmonize your submission dates. This is actually very simple! Basically, all IND updates (and foreign updates, if applicable) will be aligned with the date that the first authorization for clinical use went into effect in any region. This is also known as the Development International Birth Date (DIBD) for the drug.
And so it goes!
Admittedly, there are many ways to update an IND (and many reasons for doing so) and we’ve only covered a few of the most common possibilities here. Ultimately, over the course of a drug development program, your IND will be updated continuously to ensure that data are presented accurately and to (hopefully!) support a future marketing application. If you have any questions about IND updates, don’t hesitate to contact us: we’re happy to help!

Category: Medical Writing Services
Keywords: Investigational New Drug, Annual Report, DSUR, update
Other Posts You Might Like:
- Syner-G Solutions To Navigating Medical Writing From Home
- Shortening the Timeline: A Medical Writer’s Guide to Success
- Writing with Style: Why styles are important in medical writing
- Regulatory Medical Writing: What Is It and What Do They Do?
- Prescription for Success: Streamlining Meetings to Craft Compelling Regulatory Documents
