August 20, 2015 | Mark Cierpial, PhD, RAC, Chief Executive Officer | Regulatory Affairs
We’ve all seen or heard them – either in popular magazines and newspapers or on television and radio – advertisements directed to us, the consumers of prescription medications.
These so-called direct-to-consumer (DTC) ads have their supporters and opponents.
Some say they provide useful information to consumers, educating us on diseases we may not be aware of and promoting discussions with healthcare professionals. Others say they can easily mislead the general public about the risks of prescription drugs and promote the overuse of the expensive medications that appear in ads.
Regardless of where your opinion lies, pharmaceutical companies in the US have a first amendment right to engage in DTC advertising. Therefore, it falls to the FDA, as well as the Federal Trade Commission (FTC), to set the boundaries of what is permissible.
| Types of DTC Ads
DTC advertisements come in three flavors:
As described below, the FDA only regulates one of these three types, product claim ads. But pharmaceutical companies must be careful not to cross the line between types 1 and 2 and the FDA-regulated type 3. |
 Did you know that the United States is one of only two developed countries that allow DTC advertising of prescription drug products? Can you name the other country? Here’s a hint … Did you know that the United States is one of only two developed countries that allow DTC advertising of prescription drug products? Can you name the other country? Here’s a hint …(Answer at the bottom of the post) |
|
 |
 |
|
1. Help-seeking Ads
Help-seeking ads describe a disease or condition without recommending or suggesting a specific drug to consumers. Instead, they encourage individuals with a set of symptoms to contact their doctor.
These ads may include a pharmaceutical company’s name and phone number but may not reference a specific drug.
The FTC regulates lawful help-seeking ads.
If an advertisement mentions a drug name in conjunction with the disease or condition, it is no longer a help-seeking ad but instead becomes a product claim ad regulated by the FDA.

2. Reminder Ads
Reminder ads provide a drug’s name but not the indication(s) for which it is used. Seeing the drug name in the ad is simply meant to remind the consumer that the drug is available.
These ads are targeted at individuals who are already taking the drug, or have taken the drug in the past, and therefore already know what condition it treats, as well as its potential side effects.
Reminder ads can also introduce newly available formulations of a drug (e.g., extended-release forms) or offer incentives such as cost savings coupons.
Whereas help-seeking ads name a disease but not a drug, reminder ads name a drug but not a disease.
The FTC regulates reminder ads. However, like help-seeking ads, reminder ads cross the line into product claim ads if they mention both things together – a disease and a drug.
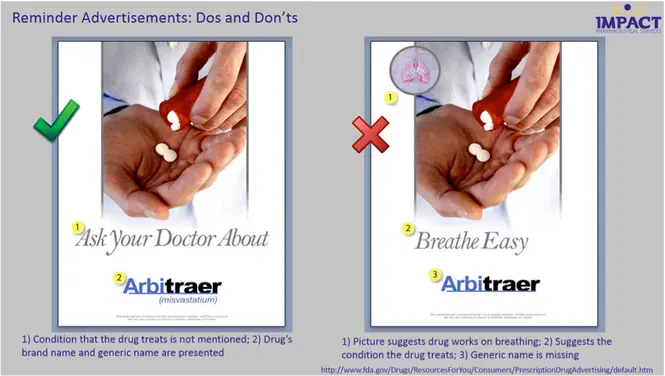
3. Product Claim Ads
By now, you’ve probably guessed the definition of a product claim ad. These ads name a specific drug along with what it treats.
The general requirements for product claim ads are:
- Must not be false or misleading
- Must present a fair balance of benefit and risk information
- Must include material facts (important to a person deciding whether or not to use the product)
- Must include a brief summary of risks
Presenting a “brief summary” of the product’s risks can be handled easily in print ads where a company can buy plenty of space. These are usually 2-3 pages long, with a balance of safety and efficacy information appearing on page 1, followed by more detailed safety information (usually in question and answer format) on page 2.
Providing this level of safety information in a TV ad, however, is not feasible due to time constraints (and attention span). For DTC TV ads, FDA allows pharmaceutical companies to satisfy the “brief summary” requirement by providing (1) a major statement and (2) adequate provision, as follows:
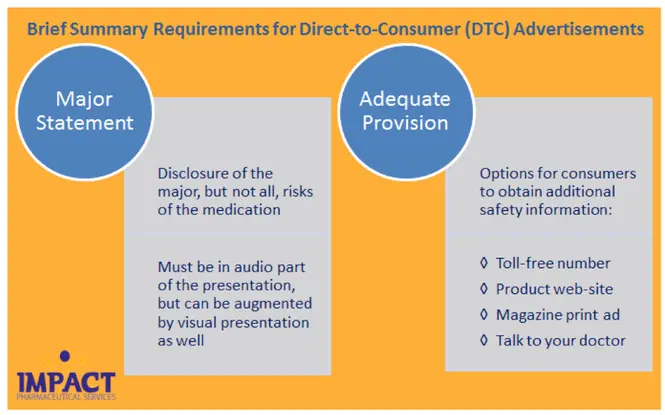
 |
Online Ads and Social Media
The regulation of pharmaceutical DTC ads became more complicated once the platform moved from print and TV ads to online, via the internet and social media. Look for a forthcoming blog from Elaina Howard on how the FDA is addressing this! |
![]()
p.s. New Zealand is the only other developed country that currently allows direct-to-consumer prescription drug advertising. And no, that’s not a map of Japan ☺
Category: Regulatory Affairs
Keywords: DTC Ads
Other Posts You Might Like:
