February 10, 2016 | Chip Carnathan, PhD, RAC, Director Regulatory Affairs | Regulatory Affairs, Drug Development Consulting
Yeah, yeah, we know. The general public considers biosimilars to be “generic” versions of approved biological products. The FDA has gone to great lengths to disavow us of this perception because, as we have discussed before, there cannot be a true generic version of a biologic product.
The FDA recently finalized a procedural guidance entitled “Formal Meetings between the FDA and Biosimilar Biological Product Sponsors or Applicants.” Since these meetings are intended to discuss the development of biosimilar products, it is somewhat appropriate that FDA chose to be “non-generic” in its design of these meetings!
Differences between BsUFA and PDUFA Formal Meetings
Formal meetings between Sponsors and the FDA on biosimilar development programs were authorized by the Biosimilar User Fee Act of 2012 (BsUFA), which was included in that year’s Food and Drug Administration Safety and Innovation Act (FDASIA). While the BsUFA meeting program is similar to the Pharmaceutical Drug User Fee Act (PDUFA) meeting program, there are some meaningful differences.
The most obvious difference is that there are 4 types of Biosimilar Biological Product Development (BPD) meetings (BPD Type 1, BPD Type 2, BPD Type 3, and BPD Type 4) instead of the more familiar Type A, Type B, and Type C meetings offered by FDA in the development of drug products.
As described in more detail later, this difference is not strictly cosmetic; however, this change is comparatively minor compared to 2 more critical differences between Sponsor-FDA meetings under BsUFA and under PDUFA.
The more critical changes under BsUFA are that:
- the FDA requires that the final meeting (briefing) package be submitted at the same time as the meeting request, and
- user fees will be assessed by FDA as soon as a Sponsor chooses to participate in the BPD program.
Meeting Types
The FDA defines 4 types of BPD meetings, as described below:
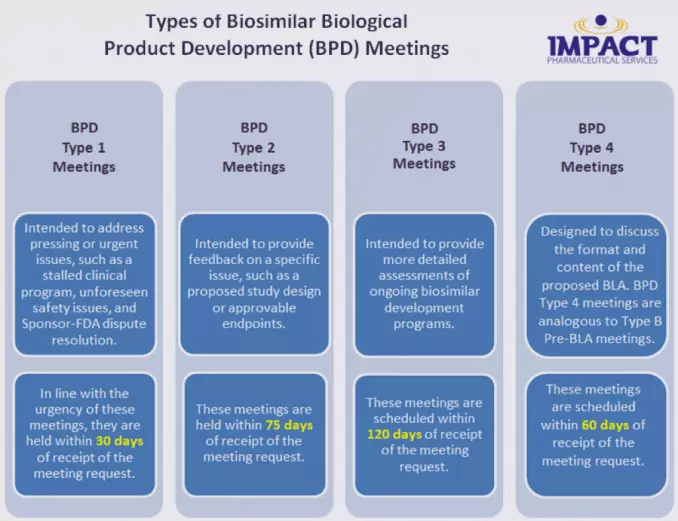
In addition to the BPD meetings, Sponsors can request a Biosimilar Initial Advisory meeting. This one‑time meeting, held within 90 days of the request, is designed to provide feedback as to the feasibility of the development of a biosimilar. During the meeting, preliminary analytical data are discussed and the proposed development program is presented. This meeting is similar to a Type B, Pre-IND meeting.
Meeting Requests
The request for a BPD Type-1, -2, -3, or -4 meeting must include adequately detailed information for the FDA to assess the need for the meeting.
The information needed is fairly lengthy, including a list of the specific meeting objectives as well as a list of the questions to be addressed by FDA. The meeting request format is very similar to that used to request Sponsor-FDA meetings under PDUFA.
Under BsUFA, however, the meeting request is incomplete and will be denied unless the request is accompanied by the submission of the final meeting (briefing) package. Details about the required contents of the meeting request and the meeting package is included in the final guidance document.
User Fees
The BsUFA stipulates that Sponsors must pay a BPD fee to participate in the BPD program (initial fee), which includes participation in the BPD meetings discussed above. The BPD fee is a per-product annual fee (ie, not a per-meeting or a per-review fee) that is assessed until the filing of a marketing application or the discontinuation of the BPD program.
 |
Both the initial and annual BPD fees are currently set at $237,420, which is not chump change!
The quid pro quo of the user fees is that they provide the revenues needed for the FDA to establish a well-managed biosimilar biological product review process and the FDA has committed to certain performance goals. |
The Biosimilar Initial Advisory Meeting is not considered to be part of the BPD program, per se, therefore a user fee is not assessed. Clearly, it is to the Sponsor’s advantage to request an initial meeting in order to get critical FDA feedback on the proposed program prior to incurring the initial and annual user fees!
Wrap-up
Although the title of the guidance (Formal Meetings Between the FDA and Biosimilar Biological Product Sponsors of Applications) is straightforward, the document is surprisingly complex.
The 4 separate meeting types are described, each with unique outcomes, review requirements, and schedule lead-in periods. The guidance provides details about the meeting request document and the meeting briefing package. Finally, user fees are introduced for programs prior to licensing application.
Full comprehension of the document will come only through real-life experience! Don’t hesitate to contact us if you need help with your biosimilar program.

Category: Regulatory Affairs, Drug Development Consulting
Keywords: Biosimilar, FDA guidance
Charges image courtesy of Stuart Miles from freedigitalphotos.net.
Other Posts You Might Like:
