February 18, 2016 | B J. Witkin, Senior Manager | Regulatory Operations
I recently attended the DIA’s Regulatory Submissions, Information, and Document Management (RSIDM) forum, an annual conference for people who work in Regulatory Operations and Regulatory Information. As usual, I learned some things and got some food for thought. I wanted to let you know about 2 really productive sessions I attended.
The future of eCTD: eCTD v4 and Regulated Product Submissions (RPS)
One of the best sessions I attended was called “eCTD v4/RPS: Moving Towards Implementation.” The session was moderated by Mark Gray of FDA—he’s always excellent—and 2 of the presenters were Jared Lantzy (formerly of FDA) and Joel Finkle, both of whom have been following RPS and eCTD v4 for years and really, really know their stuff.
What is eCTD v4?
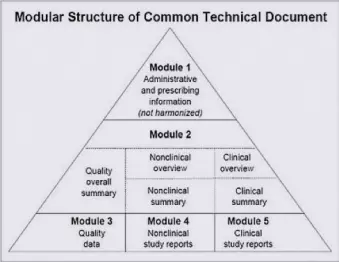 eCTD v4 is the next major update to eCTD and, if what I heard comes to fruition, it will be a major change. The good news is that the infamous eCTD pyramid isn’t going anywhere.
eCTD v4 is the next major update to eCTD and, if what I heard comes to fruition, it will be a major change. The good news is that the infamous eCTD pyramid isn’t going anywhere.
We’ll continue to have the usual 5 modules, though we may get some new headings and metadata added. The big changes will happen behind the scenes, so if you’re a geek like I am this will probably excite you.
Here is a list of the changes coming with ECTD v4 (with thanks to Jared Lantzy):
- A single XML backbone
Currently each submission has at least 2 XML files to describe the structure; eCTD v4 will have 1.
- A “lite” folder structure
The current folder structure is extremely hierarchical and that can create problems—especially in module 5. With eCTD v4 the plan is to reduce the number of folders to only a level or two.
- Study Tagging Files, Node Extensions, and the Append lifecycle operator will be removed
All of these current eCTD features are confusing, problematic, or both (in fact, the EMA didn’t implement STFs). In eCTD v4 they’re removed.
There are also several enhancements:
- Document reuse will become easier
We’ll be able to reuse a document simply by “pointing” at it, even across applications, instead of resubmitting it. I see lots of possibilities here. The biggest potential would be reusing your study reports when you file the NDA without having to resubmit them from the IND. I suppose that you could even recreate your entire module 4 and module 5 (or as much as you need to) from the IND without having to resubmit them!
- You’ll be able to specify a display order
Currently, documents in a submission or your application display in the order determined by your eCTD viewer. Now you’ll be able to tell the viewer the order in which the documents should be displayed.
- You can fix your metadata
If you made a mistake in something like the name of your indication or your drug substance manufacturer, currently you either have to live with it or resubmit your documents with the corrected metadata and delete the old ones. eCTD v4 will allow you to correct that kind of error.
- Two-way communication
We’ve been hearing about this for years but I’m skeptical whether it’ll work. As I understand it, agencies will be able to “submit” documents to your eCTD structure—correspondence, meeting minutes, etc. It sounds great and makes sense…but how would I know to go check if there were updates? Would I have to check the Electronic Submissions Gateway daily? If you’re a CRO and you have lots of Sponsors this sounds difficult. I’ve asked how the notification process would work but so far I haven’t heard a real answer. My feeling is that it’s far off enough that no one really knows exactly how it will work.
Now the bad news: it doesn’t look like eCTD v4 will be implemented for several years. Mark Gray said that we might be able to start pilot testing eCTD v4 sometime next year, but the pilot program will probably go on for a while. As it is, eCTD v3.3 which went live in May of 2015 still isn’t mandatory. Mr. Gray said that v3.3 will be made mandatory before v4 is even officially released.
The medical writer-to-publisher handoff
I was one of the presenters at a session focused on best practices for the medical writer-to-publisher handoff, where I represented the publisher perspective with 2 writers representing the medical writer perspective. As strange as it may sound, I learned some things during it!
My presentation discussed formatting standards for clinical summaries and the importance of communicating them to the medical writer. I presented examples of formatting standards which have always worked for me but several attendees shared scenarios in which these standards don’t work for them or aren’t as beneficial as other formats.
Here’s an example: currently I usually tell my writers to make external references blue so they stand out. Let’s say they do that for references to the listings and later the document is submitted to the EMA. Since listings aren’t submitted as part of the CSR to EMA, I’d now have to make them black.
Although some of the suggestions were tool- or process-specific, my takeaway was this: think about the big picture (like, will this document be submitted to other countries?) before you start a large project like an NDA.
The terrific audience and its feedback gave me a lot to think about! I’m going to ponder their suggestions so watch this space for future posts as I try things out and see how they work.
Finally, the highlight of this conference is always the FDA’s 2 presentations. I’ll post my notes from these separately so keep an eye out so you’re always in the know. And if you missed my presentation and have any questions, don’t hesitate to contact us. I’m happy to chat!

Category: Regulatory Operations
Keywords: DIA RSIDM, eCTD v4, XML backbone
Other Posts You Might Like:
