January 10, 2022 | Julia DiFiore, PhD | Clinical Research Scientist
Many of us involved with drug development derive a lot of satisfaction in knowing that our work contributes to improving the health of patients around the globe. We offer our insight and expertise to design studies, analyze the results, and hopefully one day submit everything to regulatory agencies so that patients can access the treatments they need.
But where does patient input fit into all of this? Patients are, after all, the experts on living with the disease and how it impacts their day-to-day. They are also the ultimate consumers of the products that the pharmaceutical industry produces.
What is their quality of life with current treatments, if any are available? What would they want to see in future treatments? What could be done to make participation in clinical trials easier for them? These valuable insights need to be considered when thinking about drug development so that patients benefit in meaningful ways from new therapies.
Questions like these are ones that the FDA wants to address through a series of initiatives related to patient-focused drug development (PFDD). As the name suggests, PFDD involves obtaining patient input in order to understand their perspective on the disease impacting their life, clinical assessments, and treatments, and incorporate that input into the clinical development process.
Since 2012, the FDA has put the following PFDD initiatives in place:
- Disease-specific PFDD meetings
- PFDD Methodological Guidances
- Clinical Outcome Assessments (COA) Grant Program
- Encouraging external PFDD meetings
It is exciting to see the valuable insight from the patient experience being more widely incorporated by the FDA and sponsors into the clinical development process.
With almost a decade of work on these initiatives, there is a lot to unpack. Below we cover some of the highlights of PFDD efforts from the FDA so far and discuss their impact on clinical development.
Disease-specific PFDD Meetings

Starting in 2013 under PDUFA V, the FDA began holding disease-specific PFDD meetings in order to directly hear from patients and caregivers about their experiences living with certain diseases and their perspective on current treatments.
The diseases were chosen based on thousands of public comments from Federal Register notices (77 FR 58849 and 79 FR 60857) and criteria that the FDA developed in order to capture the variety of diseases seen during regulatory review. Some of the criteria that were used to select the disease areas included:
- Disease areas that are chronic, symptomatic, or affect functioning and activities of daily living
- Disease areas for which there are currently no therapies or very few therapies, or the available therapies do not directly affect how a patient feels or functions
- Disease areas that reflect a range of severity, from diseases that are life-threatening to those that are mild and symptomatic
Ultimately, over 20 meetings were held which covered a range of disease areas such as autism, gastrointestinal disorders, and HIV. Each meeting started with an overview of the disease area and current treatment options. The meetings then moved into panel and large-group facilitated discussions. A summary of each meeting can be found in the Voice of the Patient report on the FDA website.
Patients and caregivers shared deeply personal stories and concerns during these meetings.
For example, autism patients and caregivers discussed the challenges in the lack of treatment options available. Since the underlying pathophysiology of autism is still not well understood, their only options were behavioral therapy or drugs that help manage mood, aggression, and other symptoms.
Autism patients also expressed a desire to have treatment options that would effectively alleviate symptoms that affect daily functioning while allowing them to retain their unique traits. It is a tall order, but an important one, since the best therapies are those that patients are willing and able to incorporate into their lives.
The PFDD meetings provide a forum to hear valuable patient input, but how can sponsors continue to collect patient experience data and incorporate it into their clinical development programs? New FDA guidance documents will provide some direction in this area and are discussed more below.
PFDD Methodological Guidances
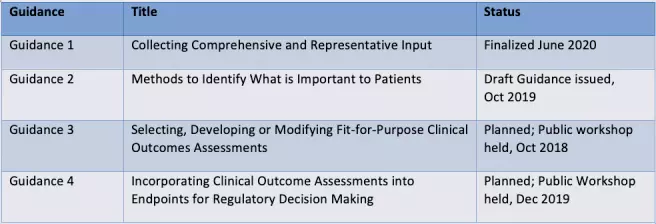
Under the 21st Century Cures Act and PDUFA VI, the FDA committed to providing guidance related to PFDD methodology in order to help sponsors better incorporate the patient perspective into clinical development programs. Below are the four guidance documents announced by the FDA and their current status:
Each guidance document provides the FDA’s current thinking on a certain aspect of PFDD, with some overlap between guidance documents.
Guidance 1 provides an overview on patient experience data and how sponsors can collect it. Given the wide variety of data that can be collected and methods to do so, this guidance aims to help sponsors think about the best data and methods for their objectives. An appendix also provides references for data standards, which sponsors can keep in mind if they plan on submitting any of the data they collect to the FDA.
Having established a broad overview of patient experience data and the advantages and limitations of specific methods to collect it in Guidance 1, Guidance 2 goes into more detail on qualitative and quantitative methods sponsors can use to determine what is important to the patient experience.
The results from patient experience studies can then be used to determine the best COA for that patient population, which will be addressed in Guidance 3. Finally, Guidance 4 will provide more detail on collecting COA data and incorporating it into regulatory decision-making.
However, since patients have already expressed that certain aspects of their disease or condition were not adequately addressed in clinical trials or current treatments at the PFDD meetings, the FDA acknowledged it will be important to establish new COAs to address these gaps in clinical testing.
Clinical Outcome Assessment (COA) Grant Program
In response to the need for new COAs, the FDA established a grant program in 2019 aimed at projects developing publicly available COAs and their associated endpoints.
For the pilot grant program, the FDA was interested in COAs and endpoints in a variety of areas including migraines, non-opioid drugs intended for the treatment of acute pain, and pediatric daily function. However, they were open to any COAs and endpoints that could potentially improve outcomes for a variety of conditions, generally encompassing the same criteria that were used to select disease areas for PFDD meetings discussed above.
COA grants for 2019 were awarded to:
- Migraine Clinical Outcome Assessment System (MiCOAS) (Vector Psychometric Group and Albert Einstein College of Medicine)
- Clinical Outcome Assessments for Acute Pain Therapeutics in Infants and Young Children (COA APTIC) (Duke University)
- Northwestern University Clinical Outcome Assessment Team (NUCOAT) for COAs related to chronic diseases affecting physical function
COA grants for 2021 were awarded to:
- Preparing a clinical outcomes assessment set for nephrotic syndrome (Prepare-NS) (University of Michigan and Northwestern University)
- Expanding the Observer-Reported Communication Ability (ORCA) Measure (Duke University)
As the development of these COAs continues, it will be exciting to see the impact they have on the clinical development process.
Encouraging External PFDD Meetings
While the FDA conducted 30 disease-specific PFDD meetings, there are still many diseases and conditions that could benefit from patient input. The FDA encourages organizations to hold their own PFDD meetings and provides considerations for planning one.
Additionally, the FDA suggests submitting a Letter of Intent (LOI) approximately 1 year prior to any meeting to explain the importance of the meeting for that specific disease or condition. The FDA will consider attending meetings, whether in person or virtually, depending on factors such as the need for better understanding the patient perspective and current staff resourcing.
The Impact and Future of PFDD
PFDD has the potential to have a major impact on drug development and we are already seeing its effects.

One example is the FDA approval of Oxlumo (lumasiran) in November 2020 as treatment for primary hyperoxaluria type I (PH1). When announcing the approval, the FDA credited the advocacy of the Oxalosis and Hyperoxaluria Foundation and Kidney Health Initiative. Oxlumo is the first approved treatment for PH1, so it is a major milestone for this patient population and their advocates!
Results of PFDD also include new disease-specific guidances that were generated after PFDD meetings, whether held by the FDA or externally. For example, a 2018 FDA-led PFDD meeting on opioid use disorder was followed by a guidance finalized in 2020 for endpoints to be included in opioid use disorder clinical trials.
Going forward, there are sure to be even more success stories surrounding PFDD. If you have any questions about PFDD that we didn’t address here or want to learn more about the services Syner-G offers, don’t hesitate to contact us!

Category: Regulatory Affairs
Keywords: Regulatory Affairs, Patient-Focused Drug Development, 21st Century Cures Act
Other Posts You Might Like:
[catlist id=47 numberposts=5 excludeposts=this]
