May 4, 2022 | Nathaniel DiTommaso, MS, Regulatory Affairs Manager
In May of 2014 and February of 2019, the FDA released final Guidance for Industry outlining the Agency’s policies and procedures regarding expedited development and review programs for new drugs and biologics intended to treat serious or life-threatening conditions.
As highlighted in these guidance documents, the FDA offers five programs: fast track designation, breakthrough therapy designation, regenerative medicine advanced therapy (RMAT) designation, accelerated approval and priority review designation. These programs are intended to facilitate and expedite development and ensure that therapies for serious conditions are approved and available to patients as soon as it can be concluded that the therapies’ benefits justify their risk. Concepts and definitions including “serious condition,” “available therapy” and “unmet medical need” are detailed in FDA’s guidance documents and are threshold criteria generally applicable for concluding if the product is a candidate for these programs.
The following tables provide an overview of the requirements, benefits, timing, and procedures to assist Sponsors and Applicants with determining the best expedited development and/or approval pathway related to their specific situation. Note that a drug or biologic may be eligible to qualify for more than one of the programs listed below. For example, a drug that has received breakthrough therapy designation or fast track designation can be eligible for the accelerated approval pathway, if relevant criteria are met.
Overview of FDA’s Expedited Development Programs
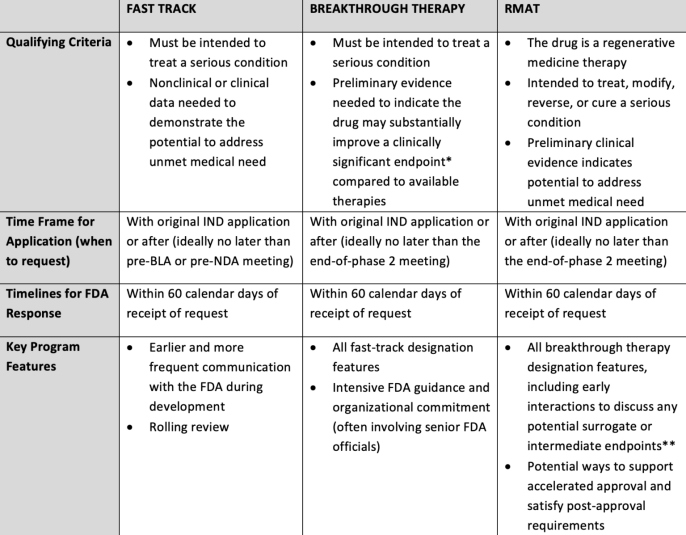
Abbreviations: BLA= Biologics License Application; IND= Investigational New Drug; NDA= New Drug Application; RMAT= Regenerative Medicine Advanced Therapy
*A clinically significant or meaningful endpoint directly measures how a patient feels, functions, or survives and represents or characterizes the clinical outcome of interest. For purposes of breakthrough therapy designation, FDA considers clinically significant endpoint generally to refer to an endpoint that measures an effect on irreversible morbidity or mortality or on symptoms that represent serious consequences of the disease.
**An intermediate clinical endpoint is a measurement of a therapeutic effect that can be measured earlier than an effect on irreversible morbidity and mortality (IMM) and is considered reasonably likely to predict the drug’s effect on IMM or other clinical benefit.
Overview of FDA’s Expedited Approval Programs
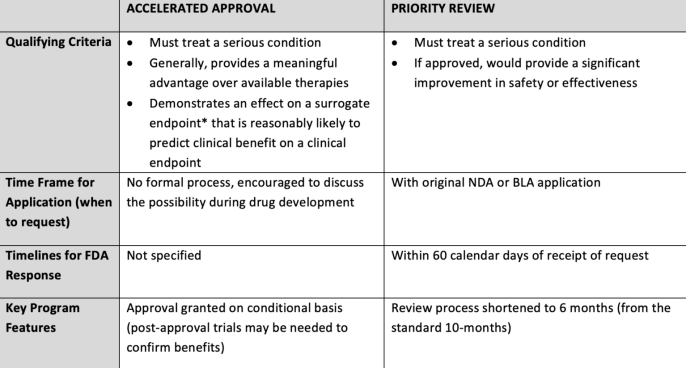
Abbreviations: BLA= Biologics License Application; NDA= New Drug Application
*A surrogate endpoint is a clinical trial endpoint used as a substitute for a direct measure of how a patient feels, functions, or survives. A surrogate endpoint does not measure the clinical benefit of primary interest in and of itself, but rather is expected to predict that clinical benefit.

The FDA is aware that patients living with serious or life-threatening conditions are in need of new promising therapies, especially when there are no satisfactory alternative therapies available. These expedited programs offer Sponsors and Applicants a way to collaborate directly with the Agency to speed the availability of new therapies to patients (e.g., timely advice and intensive guidance on clinical trial designs that may result in smaller trials that require less time to complete and/or more frequent and interactive communications with review teams), while still preserving the appropriate standards for safety and effectiveness.
Syner-G BioPharma Group understands the importance of leveraging FDA’s expedited programs to improve Sponsor chances of success and possibly shorten timelines to product approval.
Need assistance with determining if your drug or biologic qualifies for one or more of the above programs or preparing/submitting your requests to FDA? Syner-G has experienced regulatory affairs professionals that can provide you with expert consultation and strategic planning.

Category: Regulatory Affairs, Drug Development Consulting
Keywords: Expedited Development, Expedited Approval, Serious Condition, Breakthrough Therapy, Fast Track, Priority Review, Accelerated Approval, Regenerative Medicine Advanced Therapy
Other Posts You Might Like:
