Selecting a Contract Development and Marketing Organization (CDMO) is not a new topic, and much has been written and learned since CDMO’s first started to become an option in the late 1990s. If the decision is to outsource, selecting a CDMO partner remains one of the most critical and impactful decisions an organization will make for a development program. It is crucial to have a process and ability to efficiently select a CDMO, establish a partnership, and maintain a strong working relationship.
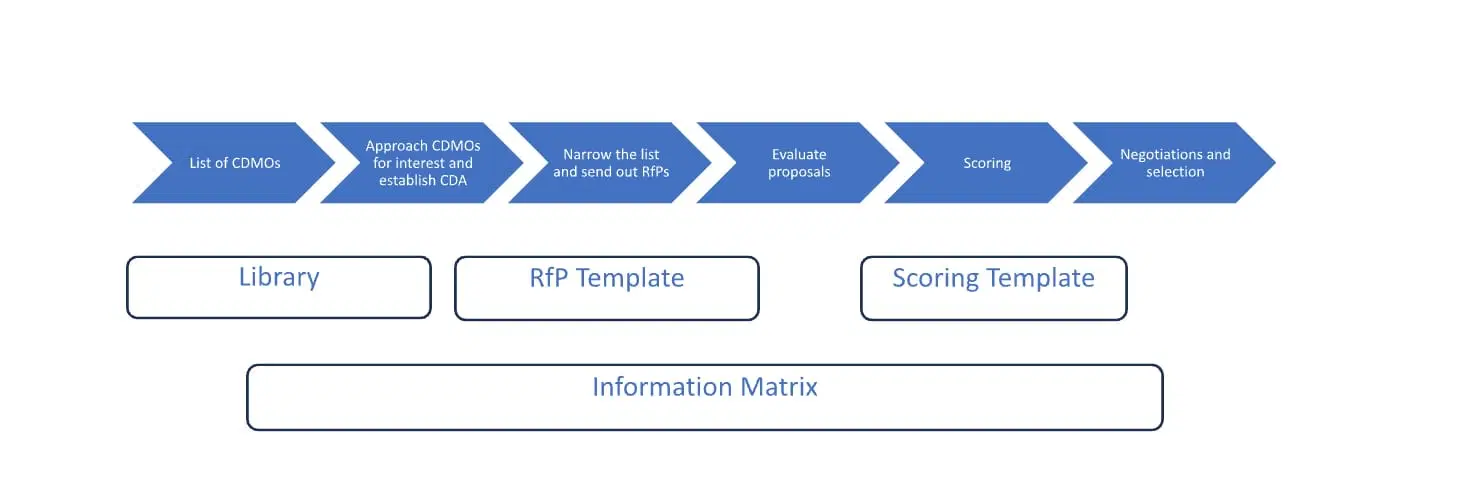
Selecting a CDMO involves a systematic approach, and utilizing a variety of tools can facilitate this approach, all helpful to drive the process and ultimately make the best possible decision. At Syner-G, four key tools leveraged in the selection process are – LIBRARY, RfP TEMPLATE, SCORING TEMPLATE and an INFORMATION MATRIX.
Figure 1: CDMO selection process and tools
A: LIST OF CDMOs
The first step in the process is to make a short list of CDMOs that you are planning to evaluate. In this step, it is helpful to have a LIBRARY of existing CDMOs with an overview of their capabilities. Start with mapping out what services you are looking to outsource (cell line development, process development, drug substance, drug product, analytical, cGMP manufacturing, scales, and technologies etc.) and determine if you want a one- stop-shop CDMO or if you prefer to use multiple CDMOs for different aspects of the program. Then, search your CDMO LIBRARY for CDMOs that have the capabilities you are looking for and make a short list.
B: APPROACH CDMOs FOR INTEREST AND ESTABLISH CDAs
Once you have created your short list of CDMOs to approach, create an INFORMATION MATRIX. This tracker is designed to easily monitor your contact interactions, recording who you reached out to, the timing of your communications, and the instances when you receive responses. There is no need to have a list of more than 10 CDMOs at this point. Initiate contact with the CDMOs you’ve listed. This can be done through direct contacts, information requests on their website, or other online contact methods. A good initial move is to set up an introductory meeting to verify their capabilities and inquire if they are open to receiving a Request for Proposal (RfP), as well as to discuss the procedure for establishing a mutual Confidential Disclosure Agreement (CDA).
C: NARROW THE LIST AND SEND OUT RfPs
In parallel with establishing CDAs, start drafting your RfP. The RfP should outline your needs, technical requirements, timelines, and general expectations. It is helpful to have an RfP TEMPLATE as a starting point, customizable for each project. After establishing CDAs and verifying that the CDMOs possess the required capabilities, proceed to distribute the RfP to the selected CDMOs. The responsiveness and communication level of CDMO representatives while setting up the CDA can serve as an initial indicator of the effectiveness of the future partnership with the CDMO..
D: EVALUATE PROPOSALS
Once you receive proposals from the CDMOs it is critical to make a like-for-like comparison. CDMOs will have their templates and definitions and work scope included in different stages can vary. At this stage, it is crucial to convene with the CDMO’s technical teams to thoroughly understand the strategy they suggest. One should plan on proposal revisions at this stage to hone in on the desired scope and to be able to more readily make a like-for-like comparison. These meetings also give you additional insight into how the CDMO is working and you can start assessing cultural fit.
E: SCORING
To objectively evaluate and drive a decision, it is helpful to do a weighted attributes evaluation using a SCORING TEMPLATE which is the 4th tool we like to use in our CDMO selection toolbox. Areas to consider include:
- Technical (capabilities, expertise, technology etc.)
- Quality/Regulatory (inspection track history, risk management, escalation etc.)
- Cost (contract terms, payment terms, costs, licensing fees etc.)
- Schedule (task durations, slot availability etc.)
- Cultural (time zone, language, geographic location, ways of working etc.)
Each area can have multiple attributes and the team should assess the weight each attribute should be assigned for the specific project. Using a scoring system helps pinpoint your top one to three candidates with whom to continue negotiations and comprehensive due diligence assessments.
F: NEGOTIATIONS AND SELECTION
Last step in the process is to negotiate contract and quality terms in the form of Master Service Agreement (MSA) and Quality Agreement (QTA).
In the dynamic landscape of biologics development, the journey to select the right CDMO is nuanced and multifaceted. Syner-G stands as a pivotal partner in this intricate process, providing not just tools but also expertise to navigate through the selection maze. With a robust track record and deep insights into the industry, our tailored approach ensures that organizations can efficiently sift through options, align strategic needs with technical capabilities, and foster collaborations that endure. As the final negotiations take place, the value of a seasoned partner like Syner-G becomes ever more apparent, transforming the daunting task of CDMO selection into a strategic advantage for biologics programs. With Syner-G, the decision to outsource becomes less of a challenge and more of an opportunity to excel and innovate within the pharmaceutical landscape.
Related Articles: Understanding Large Molecule Drug Development: From Biologics to Market