In the evolving healthcare landscape, medical writing in clinical research is a critical mainstay, seamlessly translating complex scientific discoveries into accessible, regulatory-compliant documents. This unique blend of science and communication allows breakthroughs in medical research to navigate the rigorous path from laboratory insights to real-world patient applications effectively.
By bridging the gap between dense scientific data and its practical implications, medical writing accelerates the approval process and makes it accessible to all stakeholders in the healthcare ecosystem.
Understanding Medical Writing in Clinical Research
Medical writing in clinical research involves specialized writers creating and developing scientific documents. These professionals possess a unique blend of scientific knowledge and writing expertise, enabling them to translate complex research data into clear, concise, and coherent documents. The scope of medical writing services is broad, covering everything from the documentation required for regulatory submissions to the dissemination of research findings through scientific publications.
Types of Documents Medical Writers Create in Clinical Research
Medical writers play a pivotal role in the clinical research lifecycle, producing a wide array of documents essential for the success of clinical trials and regulatory approval processes. Here’s a closer look at some of the key documents:
- Clinical Study Protocols: These documents outline the rationale, objectives, design, methodology, statistical considerations, and organization of a clinical study. They serve as a roadmap for conducting the trial, ensuring it meets scientific, ethical, and regulatory standards.
- Informed Consent Forms: Essential for ethical clinical trials, these forms provide participants with comprehensive information about the study, including its purpose, procedures, risks, benefits, and their rights as participants. The goal is to ensure that participants can make an informed decision about their involvement in the trial.
- Clinical Study Reports (CSRs): Upon the completion of a clinical trial, medical writers compile detailed reports that present the methodology, results, and conclusions of the study. These documents are critical for regulatory submissions and informing future research and clinical practice.
- Regulatory Submission Documents: These include a variety of documents required by regulatory agencies (such as the FDA in the United States) to approve new drugs, devices, and treatments. Regulatory medical writers ensure that these submissions are comprehensive and compliant to articulate the safety and efficacy of the product clearly.
- Research Articles and Abstracts: Disseminating research findings through peer-reviewed journals is key to medical writing. These articles and abstracts communicate the significance and implications of clinical research to the scientific community and beyond.
The Pivotal Role Medical Writers Play in Clinical Studies
Medical writing is crucial in clinical research as it ensures the accurate and effective communication of scientific findings, facilitating their understanding and application by the medical community and regulatory bodies. It plays a vital role in maintaining regulatory compliance, promoting patient safety, distributing research results, and advancing medical knowledge and patient care.
Enhancing Clarity and Accessibility
Medical writers are tasked with distilling complex research data into clear, concise, and accessible documents. This guarantees that findings are available and actionable across various stakeholders. They tailor information to suit the needs of diverse audiences, from laypersons to scientific experts, making the research understandable and relevant to each.
Facilitating Regulatory Approval Processes
The path to regulatory approval is laden with challenges, primarily around stringent documentation requirements. Medical writers navigate these hurdles by creating comprehensive and compliant submissions, smoothing the regulatory review process. Their in-depth understanding of international standards and guidelines makes sure that all documents meet the necessary compliance criteria, reducing the risk of delays or rejections.
Bridging the Gap
Medical writing serves as a vital bridge between research and application, ensuring that the implications of clinical studies are communicated to all involved parties, including healthcare professionals, regulatory bodies, and patients. This role in democratizing information empowers patients and the public with knowledge that can inform their healthcare decisions.
Communicating Complex Information
One of the significant challenges in clinical research is making complex information understandable to non-specialists. Medical writers excel in breaking down intricate data into engaging, digestible content. This not only simplifies the complex but also significantly extends the reach of research findings, providing the widest possible impact.
Contributing to Advancement
Medical writing directly contributes to the advancement of medical science and patient care by making sure that research findings are clearly communicated and widely understood. It plays a crucial role in publishing research articles and abstracts, sharing discoveries with the scientific community and the public. This contribution is vital for driving scientific progress and improving patient outcomes.
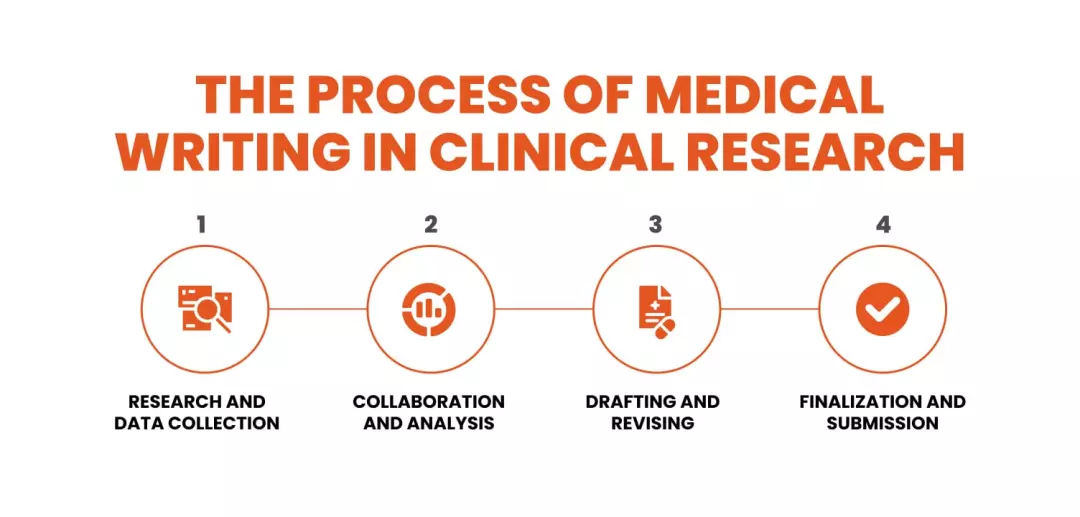
The Process of Medical Writing in Clinical Research
The medical writing process, crucial for distributing research findings, involves distinct yet interconnected stages.
Research and Data Collection
The process begins with in-depth research and meticulous data collection, where medical writers gather essential information by collaborating closely with clinical research teams.
Collaboration and Analysis
Medical writers work alongside clinical research teams to confirm documents accurately reflect study outcomes. They analyze collected data to distill the most relevant findings, a step critical for understanding the study’s implications.
Drafting and Revising
Drafting documents requires a focus on clarity, accuracy, and regulatory compliance. This phase often involves multiple revisions, incorporating feedback from peer reviews to refine the content.
Finalization and Submission
The final steps involve adhering to specific submission guidelines and ensuring documents are delivered on time. Medical writers navigate these requirements precisely, facilitating the smooth progression of clinical trials and the publication of new research.
Elevating Clinical Research Through Medical Writing
The process of medical writing is indispensable in clinical research, serving as the critical link that transforms complex scientific discoveries into clear, accessible narratives. Experienced medical writers effectively communicate valuable insights from clinical trials, support regulatory approvals, advance medical science, and enhance patient care. Medical writing is not just about documenting data; it’s about creating a pathway for discoveries to make a meaningful impact on the world.

