Navigating the regulatory landscape is a critical step in the development of biopharmaceutical products. Biologics License Applications (BLAs) and New Drug Applications (NDAs) are two distinct drug marketing applications and approval pathways designed for biologics and small-molecule drugs (traditional drug molecules), respectively.

What is a BLA?
Biologics have revolutionized modern medicine, offering highly advanced treatments like monoclonal antibodies, vaccines, and gene therapies. These therapies, derived from living organisms, have unlocked new possibilities in treating complex conditions, but their production and regulation are equally intricate.
A Biologics License Application (BLA) is the formal mechanism used to seek approval from the U.S. Food and Drug Administration (FDA) to market a biological product. This application serves as a comprehensive dossier, encompassing clinical trial data, manufacturing processes, and safety profiles. The Center for Biologics Evaluation and Research (CBER), a division of the FDA, evaluates BLAs to confirm that biologics meet the agency’s rigorous standards for safety, efficacy, and quality.
Examples of biologics submitted under a BLA include gene therapies, which repair or replace faulty genes; insulin, critical for managing diabetes; and monoclonal antibodies, a cornerstone in targeted cancer treatments. These products demonstrate the diverse potential of biologics to address unmet medical needs.
Key Requirements for a BLA
To navigate the BLA approval process, manufacturers must address several critical components.
- Description of Manufacturing Processes: Because biologics are derived from living systems, their manufacturing process is highly complex. The BLA must detail each step of production, including raw material sourcing, purification techniques, and quality control protocols. Consistency in production is essential, as even minor variations can impact the product’s performance or safety.
- Evidence of Clinical Efficacy and Safety: Robust clinical trial data must demonstrate that the biologic is both effective and safe for its intended use. This evidence includes outcomes from early-phase trials through pivotal Phase III studies, providing a comprehensive understanding of the biologic’s benefits and risks.
- Compliance with Good Manufacturing Practices (GMP): Adherence to Good Manufacturing Practices ensures that biologics are produced under stringent quality standards. Facilities must meet FDA GMP regulations to minimize risks like contamination, and ongoing inspections confirm continued regulatory compliance.
What is an NDA?
Small-molecule drugs have long been the foundation of traditional medicine, offering accessible and effective treatments for a wide range of conditions. These chemically synthesized compounds, often delivered in forms like tablets, capsules, or injectables, continue to play a crucial role in addressing everything from chronic diseases to acute illnesses.
A New Drug Application, or NDA, is the official process through which manufacturers seek marketing approval from the U.S. Food and Drug Administration to market a small-molecule drug. A standard NDA includes comprehensive data and findings from both nonclinical and clinical studies conducted on the drug. The NDA serves as a comprehensive document, consolidating data on the drug’s safety, efficacy, and manufacturing standards. The Center for Drug Evaluation and Research (CBER), a division of the FDA, oversees NDA submissions, ensuring that new drugs meet strict regulatory requirements before reaching patients.
Examples of drugs submitted under an NDA include medications for managing high blood pressure, antibiotics to treat bacterial infections, and oral antivirals for illnesses like COVID-19 or influenza.
Key Requirements for an NDA
To secure approval, an NDA must address several critical elements:
- Preclinical Data Package: This section includes data from laboratory and animal studies that examine the drug’s pharmacology, toxicology, and biological activity. These studies establish a foundation for determining the drug’s safety before advancing to human trials.
- Clinical Trial Results Demonstrating Safety and Efficacy: A detailed account of clinical trial results is a core component of the NDA. Data from all trial phases must show that the drug is effective for its intended purpose and that its benefits outweigh any associated risks. The submission should include statistically significant findings and address the needs of the target patient population.
- Labeling and Prescribing Information: The NDA must provide proposed labeling that clearly communicates the drug’s approved uses, dosing guidelines, contraindications, and potential side effects. This ensures that healthcare providers can prescribe the drug safely and effectively.
Related Article: Understanding the Phases of FDA Approval for Drug Development
Comparing BLA vs NDA
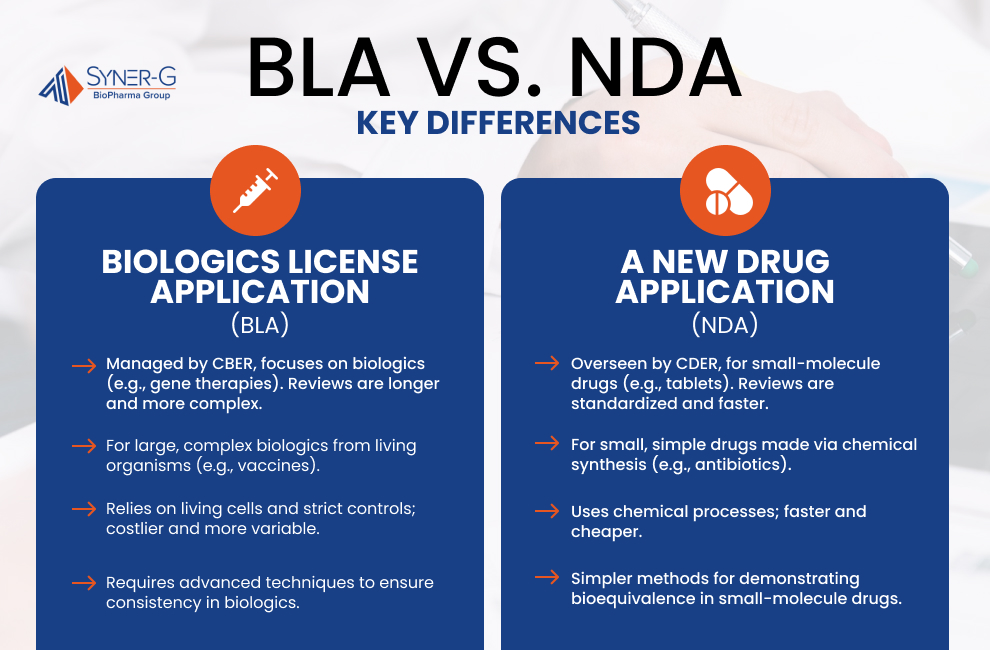
Comprehension of the distinctions between a Biologics License Application (BLA) and a New Drug Application (NDA) is crucial for navigating the regulatory landscape in biopharmaceutical development. Although both pathways are submitted to gain FDA drug approval and guarantee the safety and efficacy of therapeutic products, they cater to fundamentally different types of treatments and involve distinct requirements.
Regulatory Pathways
The regulatory pathways for BLAs and NDAs are governed by separate divisions of the FDA, each with its own focus. The Center for Biologics Evaluation and Research (CBER) oversees BLAs, which are specific to biologics, such as monoclonal antibodies and gene therapies. Conversely, the Center for Drug Evaluation and Research (CDER) handles NDAs, which cover small-molecule drugs like oral tablets or injectable solutions.
These pathways also differ in terms of review timelines and submission criteria. Biologics often require longer and more complex reviews due to the inherent variability in products derived from living systems. Small-molecule drugs, on the other hand, typically follow more standardized and predictable submission and review processes.
Product Types
The core distinction between BLAs and NDAs lies in the type of products they encompass. BLAs are designed for biologics, which are large, complex molecules derived from living organisms. These can include therapies like cell-based treatments, gene-editing products, and vaccines—especially those undergoing the FDA approval process for vaccines, which follows a distinct regulatory pathway compared to traditional drugs.
In contrast, NDAs focus on small-molecule drugs, which are typically chemically synthesized and simpler in structure. Examples include analgesics, antibiotics, and antihypertensive medications.
Manufacturing Considerations
The manufacturing processes for biologics and small-molecule drugs present vastly different challenges. Biologics require the cultivation of living cells, which necessitates stringent sterility protocols, advanced bioreactor systems, and highly controlled production environments. Any deviation in these processes can lead to variability in the final product.
In comparison, small-molecule drugs are synthesized using chemical processes that are relatively straightforward and easier to replicate. This simplicity in manufacturing contributes to faster production and lower costs associated with small-molecule drugs.
Testing and Validation
Testing and validation requirements for biologics and small molecules highlight another critical difference. For biologics, demonstrating bioequivalence or interchangeability is uniquely challenging due to the inherent variability of products derived from living systems. These tests require sophisticated analytical techniques and extensive data to confirm consistency.
Small-molecule drugs, particularly generics, benefit from more streamlined validation processes. Demonstrating bioequivalence is often more straightforward, involving well-established methods for comparing the generic drug’s pharmacokinetics to the original product.
Related Article: The Evolution of Biologic Drug Relations
Why These Differences Matter
The differences between BLAs and NDAs significantly influence development timelines, costs, and market strategies. Biologics, governed by the BLA pathway, are inherently more complex, requiring longer development timelines and higher costs due to sophisticated manufacturing and testing processes. Conversely, small-molecule drugs submitted through the NDA pathway benefit from established production methods, enabling faster timelines and lower expenses.
These distinctions also shape market strategies. Biologics often target niche markets with high unmet needs, allowing for premium pricing and extended market exclusivity. On the other hand, small molecules typically address broader markets but encounter quicker competition from generics, impacting long-term revenue potential.
The regulatory landscape is evolving with the rise of biosimilars and combination products, blurring the lines between traditional pathways. Biosimilars follow a modified BLA process to account for the complexity of biologics, while combination products, such as biologic-small molecule hybrids, require tailored regulatory approaches.
The Path to Approval
The differences between BLAs and NDAs reflect the unique challenges and opportunities of biologics and small-molecule drugs. Selecting the correct pathway depends on a thorough evaluation of the therapy’s composition, purpose, and manufacturing process. With the rise of biosimilars and combination products, understanding these distinctions is more important than ever. A well-informed regulatory strategy can streamline the approval process and help deliver groundbreaking treatments to patients efficiently.

