In today’s complex biopharmaceutical landscape, the path from discovery to market approval demands increasingly sophisticated regulatory strategies. Early-stage regulatory planning has emerged as a critical success factor, directly impacting development timelines, costs, and approval probabilities while helping organizations navigate evolving regulatory requirements across global markets.

Understanding Early-Stage Regulatory Planning
Early-stage regulatory planning represents a systematic approach to navigating the complex regulatory landscape before initiating significant drug development investments. This strategic framework encompasses regulatory requirements, scientific considerations, and business objectives to create a clear path toward market approval.
Key Components of Regulatory Strategy
A comprehensive regulatory strategy begins with thorough intelligence gathering and analyzing current guidelines across target markets while assessing precedent cases and approval pathways. This foundation enables companies to develop robust documentation plans covering chemistry, manufacturing, controls, clinical development, and safety protocols—all aligned with current regulatory requirements.
Critical to success is establishing a clear communication strategy with regulatory authorities. This includes carefully timed scientific advice meetings, pre-IND consultations, and formal milestone discussions that shape the drug development process. These interactions provide valuable insights into regulatory expectations and potential challenges before significant resources are committed.
Integration with Drug Development Timeline
During early non-clinical development and into Phase 1, companies focus on initial regulatory pathway selection and target product profile development.
As development progresses through Phase 2, strategies are refined based on regulatory feedback, with quality systems implementation becoming increasingly important.
By Phase 3, attention shifts to marketing application strategy and manufacturing compliance preparation.
This integrated approach makes sure regulatory considerations drive development decisions rather than becoming afterthoughts. When executed properly, early-stage regulatory planning significantly improves the probability of successful market authorization while reducing costly delays and development setbacks.
Companies that embrace this approach find themselves better positioned to navigate the increasingly complex biopharmaceutical regulatory landscape.
Critical Elements of Early Regulatory Planning
The success of any biopharmaceutical development program hinges on careful planning and strategic foresight from the earliest stages. Three critical elements form the foundation of effective early regulatory planning, each playing a vital role in streamlining the path to market approval.
Target Product Profile (TPP)
The Target Product Profile serves as a strategic blueprint that guides pharmaceutical development from inception through approval. A well-crafted TPP defines critical quality attributes, intended use, safety parameters, and efficacy targets that align with both market needs and regulatory expectations.
Companies must continuously refine their TPP through regulatory authority feedback and emerging clinical data, ensuring it remains a living document that guides development decisions.
Quality by Design (QbD)
QbD implementation represents a systematic approach to pharmaceutical development that builds quality into the product from the outset. This approach begins with defining the quality target product profile (QTPP) and identifying critical quality attributes (CQAs).
Through process parameter analysis and design space development, companies establish robust manufacturing processes that consistently deliver products meeting predetermined specifications.
The implementation of QbD principles significantly impacts regulatory success by demonstrating to authorities a thorough understanding of the product and process. This approach typically results in more flexible regulatory approvals, reduced post-approval changes, and faster review times.
Organizations implementing QbD effectively often experience fewer regulatory questions during review and greater confidence in their control strategy.
Risk Assessment and Mitigation
Effective risk assessment in regulatory planning involves systematic evaluation of potential challenges across the development spectrum. This includes analyzing regulatory precedents, identifying potential chemistry and manufacturing concerns, and anticipating clinical development hurdles. Companies should maintain comprehensive risk registers that track both identified and emerging risks throughout the development cycle.
Proactive risk management requires implementing targeted mitigation strategies for each identified risk. This involves developing contingency plans, establishing trigger points for mitigation actions, and maintaining open communication channels with regulatory authorities.
Organizations should regularly review and update their risk management strategies as new information becomes available, ensuring continued alignment with regulatory expectations and development goals.
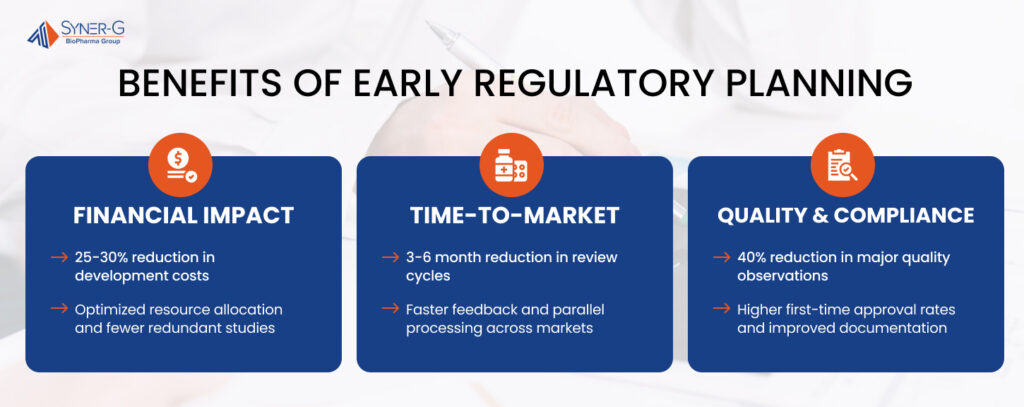
Benefits of Early Regulatory Planning
Early implementation of regulatory planning delivers measurable advantages across three critical dimensions:
Financial Impact
Early regulatory planning significantly reduces development costs through:
- 25-30% reduction in overall development costs through prevention of late-stage modifications
- Optimization of resource allocation in CMC development and clinical trials
- Minimization of redundant clinical studies through strategic planning
- Reduced need for remediation activities and post-approval changes
Time-to-Market Advantages
Strategic regulatory planning accelerates market entry via:
- 3-6 month reduction in review cycles through comprehensive submission preparation
- Faster authority feedback through strategic consultation timing
- Reduced information requests during review periods
- Parallel processing of regulatory requirements across multiple markets
Quality and Compliance
Systematic early planning enhances quality outcomes through the following:
- 40% reduction in major quality observations during inspections
- Higher first-time approval rates across global markets
- Enhanced consistency in manufacturing processes
- Improved documentation quality and regulatory compliance
These benefits create lasting organizational capabilities that extend beyond individual product commercial success, establishing sustainable competitive advantages in the biopharmaceutical marketplace.

Implementation Strategies
Implementing effective regulatory strategies requires careful orchestration of teams, tools, and processes. Success depends on establishing robust organizational frameworks that support regulatory compliance while maintaining operational efficiency.
Building Cross-functional Teams
Successful regulatory planning hinges on integrated teams led by regulatory affairs professionals who serve as strategic advisors throughout the development process. These teams coordinate across clinical development, manufacturing, and quality assurance departments through structured governance frameworks and clear communication channels.
Regular strategic alignment meetings ensure regulatory considerations inform critical development decisions while maintaining project momentum.
Regulatory affairs professionals act as both strategic advisors and tactical implementers, translating regulatory requirements into actionable development plans. Their expertise guides cross-functional decision-making, ensuring regulatory compliance without compromising development efficiency.
This collaborative approach enables early identification of potential hurdles and facilitates rapid problem-solving across organizational boundaries.
Documentation and Data Management
Modern regulatory compliance demands sophisticated documentation systems that support global submissions while maintaining data integrity.
Electronic document management systems provide the backbone for regulatory operations, enabling real-time tracking of commitments and submissions while ensuring version control across global teams. These systems integrate with quality management processes, creating a seamless flow of information from development through submission.
Organizations increasingly leverage cloud-based solutions to facilitate global accessibility while maintaining compliance with data privacy regulations. Digital tools streamline document review workflows and automate routine tasks, freeing regulatory teams to focus on strategic activities.
Regular system audits and standardized processes ensure documentation quality while meeting evolving electronic submission requirements across regulatory agencies.
The Future of Regulatory Excellence in Biopharma
Early regulatory planning represents more than just a compliance strategy—it’s a fundamental driver of biopharmaceutical success in today’s complex development landscape. Organizations that embrace comprehensive regulatory planning from the earliest stages position themselves for accelerated approvals, optimized resource utilization, and enhanced market competitiveness.
As the pharmaceutical industry continues to evolve, pharma companies that maintain adaptable regulatory strategies while leveraging modern tools and cross-functional expertise will lead the next generation of therapeutic innovations.

