Understanding how CMC consulting accelerates product development timelines is paramount in today’s fast-paced pharmaceutical landscape, accelerating drug development timelines is essential to bringing life-saving treatments to patients sooner. Chemistry, Manufacturing, and Controls (CMC) consulting provides strategic oversight across each stage of development, guaranteeing quality and regulatory compliance while reducing costly delays.

Understanding CMC in the Drug Development Process
In the development of a drug, CMC is key to defining and controlling the quality, safety, and efficacy of the drug product. CMC covers everything from raw material sourcing and formulation development to process validation and final quality control. By setting standards at every stage, CMC makes sure that the final product meets regulatory and quality expectations, which is critical for patient safety and product performance.
CMC is deeply connected to regulatory requirements. Regulatory bodies like the FDA and EMA require CMC documentation as part of the drug approval process, covering every stage of development from preclinical studies to commercial manufacture. This documentation shows a company can manufacture a product to quality specifications, minimizing product variability or contamination. For companies navigating the complex regulatory landscape, understanding and integrating CMC requirements early can prevent costly delays and setbacks as products move from lab-scale to larger clinical trials and ultimately to full-scale manufacture.
CMC Consulting helps pharmaceutical companies align product specifications, process design, and regulatory compliance from the start. An experienced CMC consultant will ensure every step of development is optimized for consistency, quality, and scalability before submitting your IND (Investigational New Drug application). This is critical: by setting specifications and protocols that meet regulatory expectations early in development, companies can avoid the need for later changes, which can cause delays and costs. With CMC consulting, companies can move through each stage of development with confidence, knowing they have a solid strategy in place that supports regulatory compliance and efficient manufacturing.
CMC Consulting Across All Stages of Development
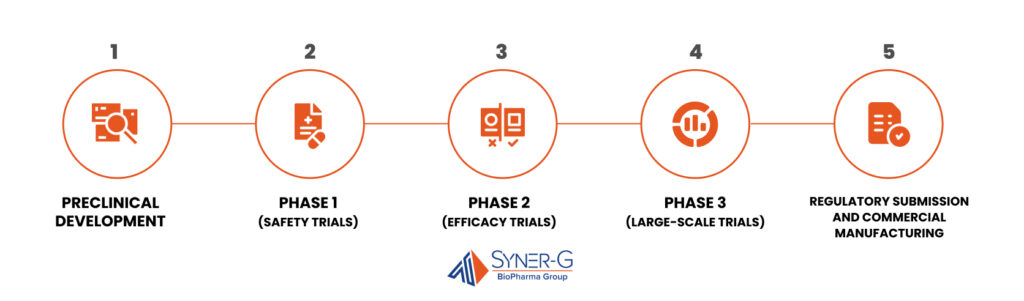
CMC Consulting is involved in every major development stage, helping streamline quality and regulatory compliance. Below is an overview of the key stages of development where CMC plays a critical role and how CMC consulting can help:
- Preclinical Development: Establishes the initial formulation, conducts stability studies, and develops analytical methods to confirm drug quality and performance. Provides critical support for raw material selection, formulation refinement, and initial quality specifications, helping to avoid issues in later phases.
- Phase 1 (Safety Trials): Develops a scalable, quality-compliant manufacturing process while confirming drug safety in a small group of volunteers. Early process optimization by CMC consultants minimizes future adjustments, supporting a smoother development path.
- Phase 2 (Efficacy Trials): Scales up production to meet the demands of larger trials, maintaining quality and regulatory compliance. Refines formulation and analytical methods as batch sizes increase, preparing the manufacturing process for subsequent stages.
- Phase 3 (Large-Scale Trials): Finalizes quality specifications and validates the manufacturing process at a commercial scale, establishing robust controls that ensure the drug’s quality and safety. Prepares the process for regulatory submission, helping avoid delays.
- Regulatory Submission and Commercial Manufacturing: Prepares and submits comprehensive CMC documentation to demonstrate product quality and manufacturing validation for regulatory approval. Supports commercial manufacturing by maintaining quality controls and handling post-approval requirements.
How Strategic CMC Consulting Streamlines Each Development Stage
Preclinical Development
In preclinical development, CMC consultants focus on early-stage formulation, stability testing, and developing analytical methods that establish the foundation for a consistent product. Setting clear quality benchmarks and robust testing methods prevents potential issues from arising in later stages.
Phase 1 Clinical Trials – Ensuring Early-Stage Safety and Quality
During Phase 1, establishing a reliable, small-scale manufacturing process is essential. CMC consultants optimize early-stage production to align with quality standards while keeping it scalable for future phases of the drug development process. Consistency in the production of clinical trial materials helps minimize the need for rework, setting up a smoother transition to larger trials.
Phase 2 Clinical Trials – Scaling Up for Efficacy Studies
As production requirements increase in Phase 2, CMC consultants support the scale-up of manufacturing processes while maintaining quality and regulatory compliance. They also refine formulation and analytical methods to address any new challenges that arise with larger batch sizes.
Phase 3 Clinical Trials – Preparing for Commercial Scale-Up
Phase 3 trials require production at near-commercial levels. CMC Consulting helps finalize process validation, quality specifications, and regulatory compliance, ensuring readiness for commercial manufacturing. This proactive preparation minimizes potential delays after Phase 3, streamlining the path to market.
Regulatory Submission and Approval
For regulatory submission, CMC consultants compile comprehensive documentation that meets all quality and compliance standards. A well-structured CMC package supports a faster review and approval process, facilitating a quicker path from trials to market and ultimately helping bring the product to patients sooner.

CMC Consulting to Speed Up Time-to-Market
CMC Consulting helps to accelerate drug development by addressing potential issues upfront. Here’s how it helps to reduce time-to-market:
Minimizing Delays Due to Manufacturing or Quality Issues
Strategic CMC planning and expert oversight identify and prevent common manufacturing mistakes such as batch quality inconsistencies or raw material issues. Addressing these upfront allows CMC consultants to avoid rework, which can take weeks to fix and delay progress.
Minimizing Regulatory Setbacks
A full CMC approach makes sure all regulatory requirements are met before submission. Preparing detailed documentation and following regulatory standards upfront enables CMC consultants to reduce the risk of setbacks during FDA or EMA reviews, avoid long regulatory delays, and support faster market access.
Simplifying Product Scale-Up for commercialization
The transition from clinical trials to full commercial manufacturing can be tricky in maintaining quality and consistency. CMC consultants simplify this process by optimizing scale-up procedures so quality standards are maintained as production increases. This upfront support reduces scale-up issues and smooths the path to commercialization and time-to-market.
Choosing the Right CMC Consulting Partner
When choosing a CMC consulting partner, Syner-G BioPharma stands out for its specific expertise, broad regulatory knowledge, and speed of the market. Syner-G BioPharma has deep knowledge across many therapeutic areas, so every CMC strategy is tailored to the specific needs and challenges of the molecule in development. Syner-G BioPharma has a proven track record of helping clients navigate the regulatory maze and minimizing risks so every stage of development meets regulatory requirements seamlessly.
One of the benefits of working with Syner-G BioPharma is their collaborative approach. They work closely with in-house teams to facilitate communication, real-time process optimization, and compliance. This supports the whole drug development process so companies can address issues proactively and stay on track for faster time-to-market.
For companies looking for a consulting partner to support their CMC strategy, Syner-G BioPharma has the expertise, reliability, and flexibility to deliver results.
Accelerate Your Path to Market with Strategic CMC Consulting
With the right CMC consulting partner, companies can streamline each phase of drug development, confirming quality, regulatory compliance, and efficiency from start to finish. CMC consultants play a crucial role in accelerating time-to-market by proactively addressing potential manufacturing, quality, and regulatory challenges. Syner-G BioPharma’s expertise and collaborative approach provide the support needed to bring innovative therapies to patients sooner. For companies aiming to expedite development while meeting high regulatory standards, Syner-G BioPharma is a trusted partner in achieving successful, timely outcomes.

