For biopharmaceutical companies, successfully completing Phase 3 clinical trials represents a pivotal milestone in drug development. However, this achievement marks not the end but rather the beginning of the final and often most scrutinized stage of the regulatory approval process.
The period between Phase 3 completion and obtaining Food and Drug Administration (FDA) approval can significantly impact strategic planning, investor relations, and ultimately, patient access to potentially life-changing therapies.
Understanding the timeline expectations and factors influencing how long FDA approval takes after Phase 3 is essential for stakeholders throughout the biopharmaceutical ecosystem.
Standard FDA Review Timeline
New Drug Application (NDA) Preparation and Submission
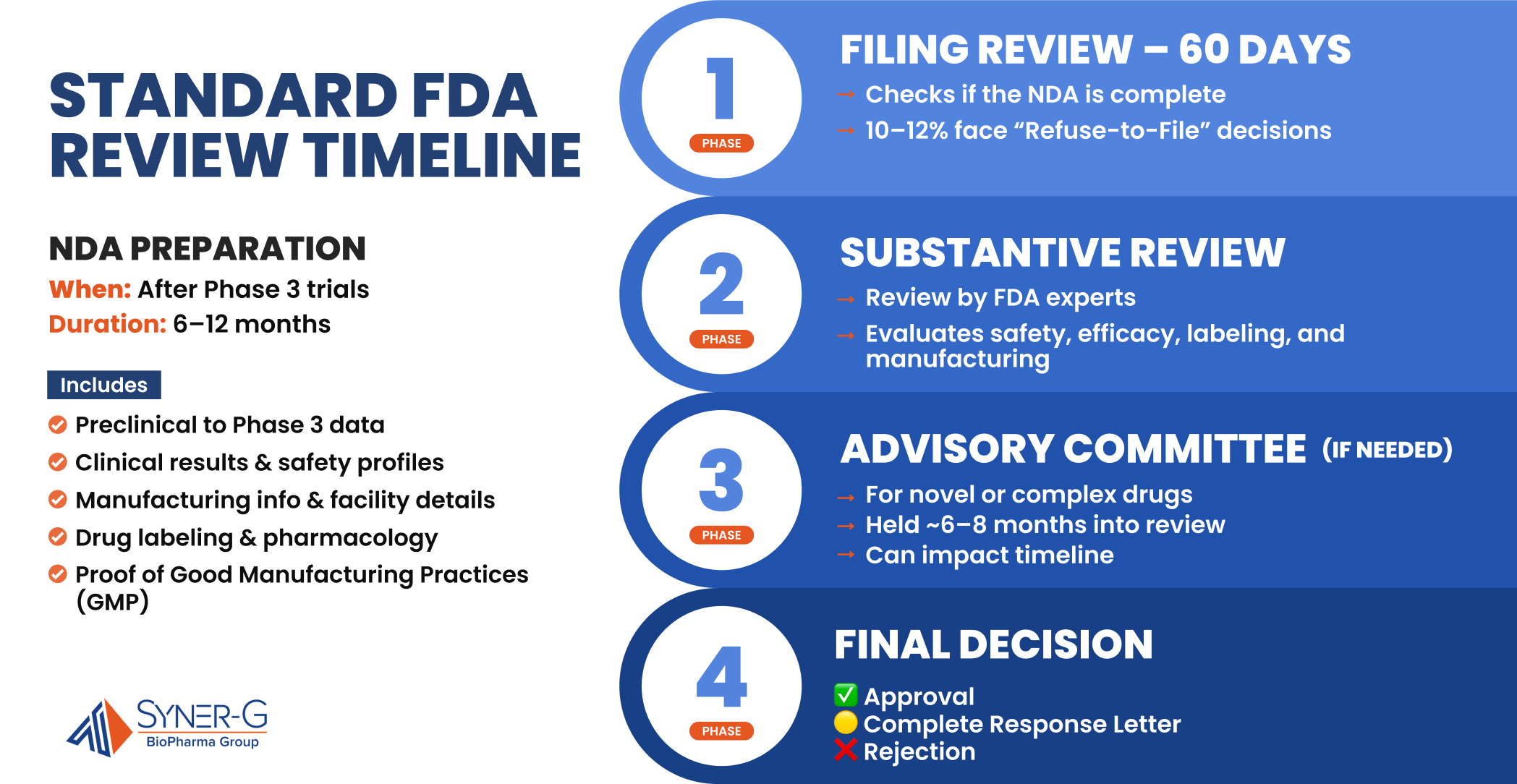
After completing Phase 3 clinical trials, sponsors must compile comprehensive documentation for a New Drug Application (NDA) or Biologics License Application (BLA). This critical step in the drug approval process typically requires 6-12 months of intensive effort to assemble all the safety and effectiveness data generated throughout preclinical research and clinical trial phases.
The NDA submission represents the culmination of years of drug development and must include all the data from preclinical studies through Phase 3, a detailed analysis of clinical data, proposed labeling, manufacturing information, and the drug’s pharmacological profile. Sponsors must demonstrate adherence to good manufacturing practices and provide extensive documentation on manufacturing facilities. The thoroughness and organization of this submission package significantly influences the subsequent review timeline.
Standard Review Process and Timelines
Under the Prescription Drug User Fee Act (PDUFA), the FDA commits to specific review timelines for new drug applications. For standard review, the FDA targets a 10-month review period from the acceptance of the NDA submission. This review process encompasses several stages:
- Filing Review (60 days): The FDA conducts an initial assessment to determine if the application is sufficiently complete to permit substantive review. Approximately 10-12% of applications receive a “refuse-to-file” decision, requiring additional data before reconsideration.
- Substantive Review: FDA review teams comprising multidisciplinary experts evaluate the submitted data regarding the new drug’s safety, efficacy, and quality. This includes examination of clinical data, adverse events, chemistry, manufacturing controls, and proposed labeling.
- Advisory Committee Meetings (if needed): For novel therapies or applications with significant scientific questions, the FDA may convene an advisory committee of independent experts. These meetings typically occur 6-8 months into the review process and can impact the final approval timeline.
- Final Decision: The culmination of the review process results in one of three outcomes: approval, complete response letter (detailing deficiencies requiring correction), or rejection.
Recent data indicates that the median time from NDA/BLA submission to FDA approval is approximately 12 months for standard review applications, with about 75-80% of drugs ultimately approved after their first review cycle. The first-cycle approval rate dipped during the pandemic years but has rebounded somewhat.
Expedited Programs: Accelerating the Approval Timeline
For drugs addressing significant unmet medical needs, the FDA offers several expedited programs that can substantially reduce how long FDA approval takes after Phase 3.
Priority Review Designation
Priority review designation means the FDA commits to an expedited 6-month review timeline instead of the standard 10 months. This designation is granted to applications for drugs that would significantly improve the treatment, diagnosis, or prevention of serious conditions. Historically, oncology products and drugs addressing rare diseases have been common recipients of priority review.
According to FDA statistics, approximately 25% of new molecular entities receive priority review designation, with average approval times of 8 months compared to 12 months for standard review.
Breakthrough Therapy and Fast Track Designations
While not directly altering the post-Phase 3 review timeline, Breakthrough Therapy and Fast Track designations facilitate more intensive FDA interaction with the sponsor during drug development, potentially resulting in more robust NDA submissions and smoother review processes. These designations often correlate with increased approval rates and shorter review times, with median approval times approximately 3 months faster than standard applications.
Accelerated Approval Pathway
The accelerated approval pathway allows drugs for serious conditions that fill an unmet medical need to be approved based on surrogate endpoints—markers that predict clinical benefit but are not direct measures of clinical benefit themselves. While this pathway may expedite initial approval, sponsors must conduct post-approval confirmatory trials to verify the anticipated clinical benefit. Cancer drugs and treatments for rare diseases frequently utilize this pathway.

Factors Influencing FDA Approval Timelines
Multiple variables can extend or compress the time from Phase 3 completion to FDA approval, making the process somewhat unpredictable despite established targets.
Quality and Completeness of Submission
Perhaps the most significant determinant of review efficiency is the quality and completeness of the NDA submission. Applications with well-organized data, thorough analysis of safety and efficacy data, clear documentation of manufacturing processes, and proactive identification of potential concerns typically navigate the review process more efficiently.
Conversely, incomplete submissions with data gaps, statistical inconsistencies, or manufacturing quality concerns often face delays or complete response letters requiring additional information.
Complexity of the Drug and Indication
Novel therapeutic modalities, first-in-class mechanisms, or complex biological products generally undergo more intensive scrutiny than drugs with well-established mechanisms or those in familiar therapeutic classes. Similarly, applications for drugs targeting complex diseases or conditions with limited treatment options may receive more extensive evaluation of risk-benefit profiles.
Recent analysis of FDA approval times indicates that drugs for rare diseases typically receive approval about 3.5 months faster than those for more common conditions, reflecting both the impact of expedited programs and the FDA’s commitment to addressing unmet medical needs.
FDA Resource Allocation and Review Capacity
The FDA’s review capacity and workload can impact timelines despite PDUFA commitments. During periods of high submission volume or public health emergencies, review resources may be stretched, potentially affecting timelines for applications without special designations.
Conversely, applications in therapeutic areas identified as high priority by the FDA may receive additional attention and resources.
Post-Submission Communication and Information Requests
During review, the FDA frequently issues information requests requiring sponsors to provide additional data clarification or analysis. The speed and quality of sponsor responses directly impact review progression. Applications requiring multiple rounds of clarification typically experience longer review cycles. Proactive anticipation of potential FDA questions and rapid, comprehensive responses facilitate smoother review processes.

Planning for Post-Phase 3 Success
For biopharmaceutical companies, strategic planning around the FDA review period is crucial for optimizing resource allocation and market preparation. Recommended strategies include:
- Early FDA Engagement: Utilizing pre-NDA meetings to align on expectations, identify potential review issues, and optimize submission strategy.
- Submission Readiness Assessment: Conducting a thorough internal review and gap analysis before submission to identify and address potential deficiencies.
- Cross-Functional Integration: Ensuring seamless coordination between clinical, regulatory, manufacturing, and commercial teams throughout the submission and review process.
- Scenario Planning: Developing contingency plans for various review outcomes, including strategies for addressing potential complete response letters or approval with post-marketing requirements.
Charting the Final Regulatory Voyage
The journey from Phase 3 completion to FDA approval represents a critical period in drug development requiring careful navigation of complex regulatory processes. While standard timelines provide useful benchmarks, the actual duration of FDA approval after Phase 3 varies significantly based on application quality, therapeutic complexity, designation status, and FDA resource allocation.
As the regulatory landscape continues to evolve, biopharmaceutical companies must maintain awareness of emerging trends in FDA priorities, review processes, and expectations regarding data quality and submission standards. Through strategic planning, proactive engagement with regulatory authorities, and meticulous attention to submission quality, sponsors can optimize their path through this rigorous process and ultimately deliver innovative therapies to patients with greater efficiency and predictability.
The interface between scientific innovation and regulatory assessment will remain dynamic, with both biopharmaceutical developers and regulatory authorities continuing to refine approaches that balance thorough evaluation with the urgency of addressing unmet medical needs.

