The medical device industry has learned a hard truth over the past decade. Even the most technologically advanced devices can fail catastrophically if they don’t account for how real people use them in real clinical situations. Healthcare providers work under immense pressure, often juggling multiple patients while making split-second decisions that can mean the difference between life and death. When medical devices don’t align with natural human behavior patterns, the results can be devastating.
This is where human factors in medical devices become critical. Understanding how users interact with technology—especially under stress—reveals risks that engineering alone cannot predict. Poorly designed interfaces, unclear alerts, or non-intuitive controls can all lead to serious errors, even when devices function perfectly from a technical standpoint.
Medical device development has traditionally focused on engineering excellence and regulatory compliance. While these elements remain crucial, they represent only part of the equation. The human element introduces variables that pure engineering cannot address. How healthcare providers perceive information, process it under stress, and translate it into action determines whether sophisticated technology actually improves patient outcomes or creates new hazards.
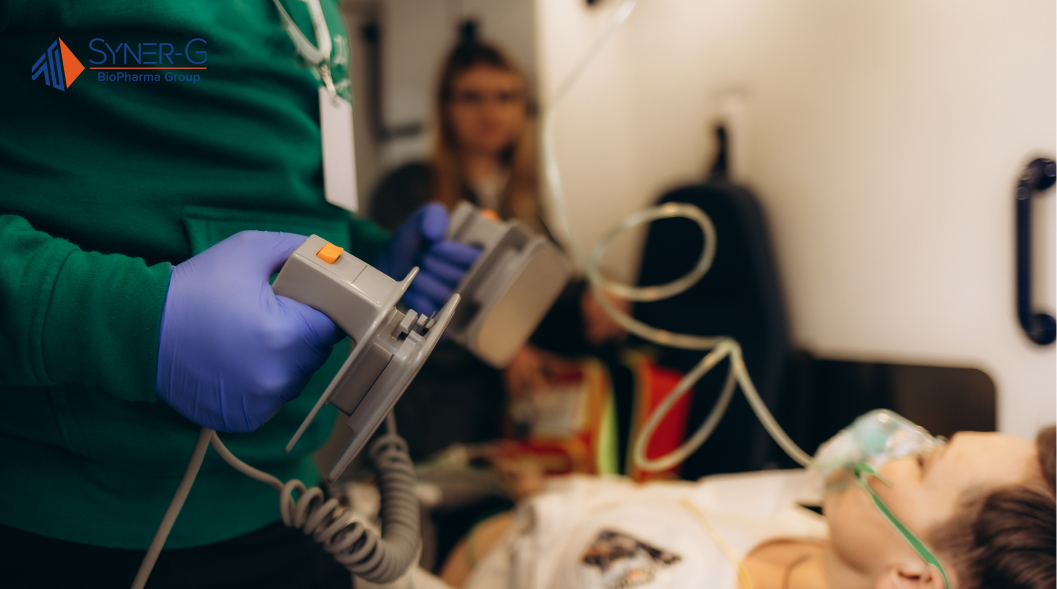
The Reality of Clinical Environments
Walk into any emergency department or intensive care unit, and you’ll witness the complex dance between humans and technology. Nurses monitor multiple patients simultaneously while responding to alarms from various devices. Physicians interpret data streams from several sources while coordinating treatment plans. Respiratory therapists adjust ventilator settings while observing patient responses and communicating with the care team.
This environment presents unique challenges for medical device design. Ambient noise levels fluctuate dramatically. Lighting conditions vary throughout the day. Space constraints force users to interact with devices from awkward angles. Interruptions occur constantly as clinical priorities shift. The patient’s current medical condition may change rapidly, requiring immediate device adjustments.
Traditional laboratory testing cannot fully replicate these conditions. Users behave differently when they know they’re being observed in controlled settings. The pressure of actual patient care creates cognitive demands that affect attention, memory, and decision-making processes. Medical device design must account for these realities rather than assuming ideal operating conditions.
How Users Actually Interact with Medical Technology
Healthcare providers develop mental models of how devices should work based on their previous experiences. These mental models influence expectations about interface layout, control placement, and feedback mechanisms. When devices violate these expectations, users experience confusion that can lead to errors. Understanding these cognitive patterns is essential for creating intuitive interfaces.
Information processing happens rapidly in clinical settings. Visual displays must convey critical information at a glance. Auditory alerts need to communicate urgency levels clearly. Tactile feedback provides confirmation that user actions have registered. The device user relies on all these sensory channels to maintain situational awareness while performing other clinical tasks.
User interface design affects how quickly healthcare providers can identify problems and respond appropriately. Poor design choices create bottlenecks in information flow. Complex navigation structures slow down critical tasks. Ambiguous status indicators force users to spend precious time interpreting device feedback. Every second of delay potentially impacts patient outcomes.
Common Patterns in Use-Related Errors
Medical device manufacturers have identified recurring themes in how use errors occur. These patterns emerge across different device categories and clinical specialties, suggesting fundamental issues with how humans and technology interact under pressure.
- Alarm fatigue develops when devices generate too many alerts, leading healthcare providers to ignore or disable important warnings
- Mode confusion occurs when users lose track of device operating states, particularly with complex systems that have multiple operational modes
- Confirmation bias causes users to see what they expect to see rather than what displays actually show, especially during high-stress situations
- Skill-based errors happen when experienced users rely on automatic behaviors that may not apply to unfamiliar device configurations
- Knowledge-based mistakes emerge when users lack complete understanding of device capabilities or limitations in specific clinical scenarios
These error patterns highlight the importance of designing devices that support natural human cognitive processes rather than working against them. Effective design anticipates where users might struggle and provides appropriate safeguards or guidance.
The Role of Contextual Research in Device Development
Understanding how medical devices perform in actual clinical environments requires research methods that go beyond traditional laboratory studies. Contextual inquiry allows researchers to observe authentic user behavior in real healthcare settings. This approach reveals insights that controlled testing cannot capture.
Healthcare providers often develop workarounds for device limitations. These adaptations may improve workflow efficiency, but sometimes introduce unintended risks. Observing these behaviors helps identify areas where device design could better support user needs. It also reveals gaps between intended use and actual use patterns that could affect safety.
User research must account for the diversity within healthcare provider populations. Experience levels vary dramatically even within the same role category. Newer staff members may rely more heavily on device interfaces for guidance, while experienced users might prefer streamlined interactions that minimize steps. Age-related factors can affect vision, dexterity, and technology comfort levels.

Building Better Design Processes
The medical device design process has evolved to incorporate user feedback much earlier in development cycles. This shift prevents costly redesigns later when problems become more difficult and expensive to address. Early user involvement also increases the likelihood that final products will meet real clinical needs rather than assumed requirements.
Usability testing protocols have become more sophisticated in their ability to simulate realistic use conditions. Testing scenarios now incorporate typical clinical distractions and time pressures. Multiple user groups participate to ensure that device interfaces work effectively across different experience levels and clinical roles.
Risk management approaches now systematically evaluate use-related hazards alongside traditional engineering risks. This comprehensive view identifies potential failure modes that could arise from human-device interactions. Design controls ensure that identified risks receive appropriate mitigation through interface modifications, user training enhancements, or procedural safeguards.
The Impact of Regulatory Requirements
FDA guidance documents have formalized expectations for addressing human factors in medical device development. These requirements mandate systematic evaluation of user needs and validation that final designs successfully address identified requirements. The regulatory framework provides structure for what was previously an inconsistent industry practice.
Quality system regulations require ongoing monitoring of device performance in real-world use. This surveillance captures use-related problems that may not have emerged during premarket testing. Post-market data feeds back into design improvement processes for future product generations.
Medical device manufacturers now invest significantly in human factors expertise. This specialization brings scientific rigor to understanding user behavior and translating insights into design improvements. Human factors professionals bridge the gap between engineering capabilities and clinical realities.
Related Article: The Next Generation of Regulatory Submissions
Adapting to Tomorrow’s Healthcare Landscape
The medical device industry continues evolving its approach to human-centered design. New technologies create opportunities for more intuitive interfaces while also introducing novel categories of potential use errors. Artificial intelligence systems may reduce some types of human error while creating new dependencies on algorithmic decision-making.
Healthcare delivery models are changing in ways that affect how medical devices are used. Telemedicine expands device use beyond traditional clinical settings. Home healthcare increases reliance on the patient and caregiver to operate sophisticated equipment. These trends require broader thinking about who constitutes the intended user population.
The fundamental challenge remains constant, though. Medical devices must work reliably when operated by real people under real clinical pressures. Success depends on understanding human capabilities and limitations, then designing technology that enhances rather than complicates clinical care. This human-centered approach ultimately determines whether medical innovation truly improves patient outcomes.

