Understanding the intricacies of navigating regulatory affairs for clinical trials is pivotal in shaping the path to successful medical research and innovation. These regulations form the backbone of ensuring that your trials are not only compliant but also set up for approval from the onset.
Key Regulatory Bodies and Their Roles
Navigating through the world of clinical trials requires a thorough understanding of the roles and responsibilities of key regulatory bodies. These organizations are critical in setting standards and guidelines for the safety, efficacy, and ethical management of clinical research globally.
Food and Drug Administration (FDA)
The FDA is a central figure in the United States. Its job is to oversee the development and approval of pharmaceuticals, medical devices, and biologics. The Agency’s primary role in clinical trials is to protect participants and ensure that the data generated are credible and accurate, which helps determine whether a drug or device can be approved for market release.
The FDA mandates strict adherence to Good Clinical Practice (GCP), which includes regulations covering the design, conduct, monitoring, and reporting of clinical trials. These are detailed in the Code of Federal Regulations Title 21 and FDA Guidance documents, which together provide a robust framework for maintaining trial integrity and compliance.
European Medicines Agency (EMA)
In Europe, the EMA mirrors some of the FDA’s functions but operates within a different regulatory framework. The Agency is responsible for the scientific evaluation of applications for marketing authorization across EU member states, directly or through a network of national competent authorities. It plays a crucial role in ensuring that all medicinal products sold on the European market are safe, effective, and of high quality.
For most products, the EMA uses a centralized procedure [KM1] that allows for simultaneous marketing authorization in all EU countries.
The different approaches across FDA and EMA can lead to differences in how clinical trials are conducted and regulated between the two regions.
Other Global Regulatory Authorities
Beyond the FDA and EMA, each region globally has its own regulatory agencies with distinct roles, such as the Central Drugs Standard Control Organization (CDSCO) in India, the National Medical Products Administration (NMPA) in China, Health Canada, the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom, the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, and the National Health Surveillance Agency (ANVISA) in Brazil. These bodies ensure that clinical trials conducted within their jurisdictions adhere to local laws and international standards.
The diversity of regulatory landscapes across regions like Asia and Latin America offers unique challenges and opportunities, such as diverse genetic pools and faster recruitment rates. However, these benefits come with the need for a deep understanding of regional differences in regulatory compliance to ensure successful and lawful clinical trial operations.
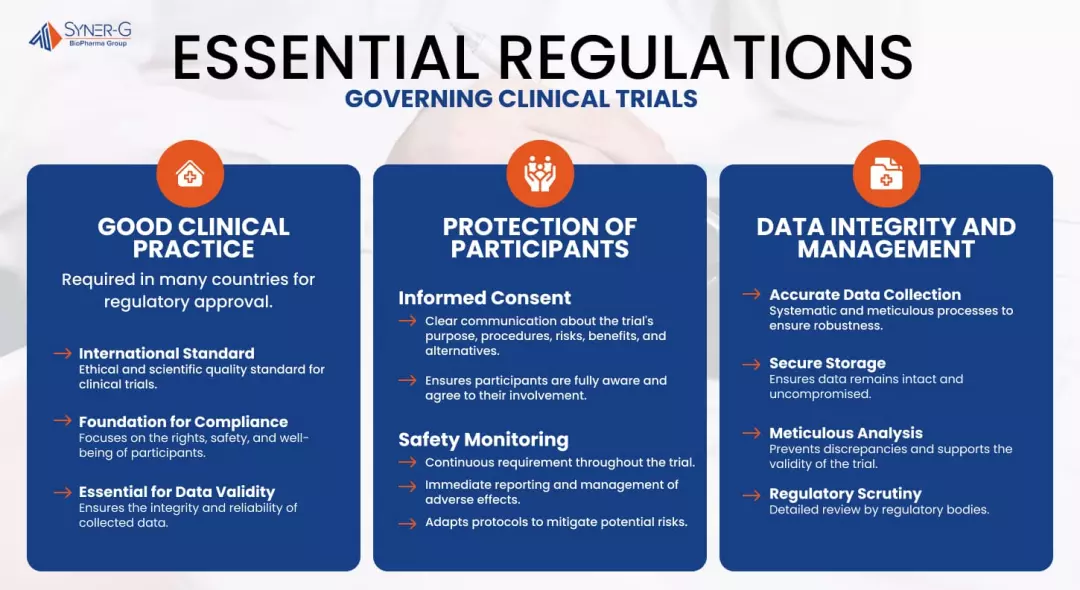
Essential Regulations Governing Clinical Trials
Navigating the landscape of clinical trials requires strict adherence to a set of comprehensive regulations designed to safeguard participant welfare, ensure data integrity, and uphold ethical standards across all research activities.
Good Clinical Practice (GCP)
Good Clinical Practice is an international ethical and scientific quality standard for conducting clinical trials. It serves as the foundation for regulatory compliance, focusing on the rights, safety, and well-being of trial participants. GCP standards and guidelines must be followed by researchers, sponsors and investigators conducting clinical trials to ensure the validity and integrity of data collected.
GCP guidelines are crucial for the reliability of clinical trial data and are mandatory in many countries. They also establish the norm for regulatory approval, enhancing public trust in pharmaceutical and therapeutic advancements. Following GCP guarantees that clinical data is both credible and reproducible, which is essential for gaining the confidence of regulatory authorities worldwide.
Protection of Clinical Trial Participants
The protection of clinical trial participants is enforced through rigorously defined regulations that prioritize informed consent, ongoing safety monitoring and protection of patient data. Informed consent is a process that requires clear communication about the trial’s purpose, procedures, risks, benefits, and alternatives. This is so that participants are fully aware and agree to their involvement under clearly stated conditions.
Furthermore, safety monitoring is a continuous requirement throughout the trial duration, necessitating immediate reporting and management of any adverse effects. This proactive approach to monitoring helps to maintain the highest safety standards, adapting protocols as necessary to mitigate any potential risks to participants.
Data Integrity and Management
Data integrity and management are critical to the success of clinical trials and subsequent regulatory approvals. Accurate and systematic data collection, paired with secure storage and meticulous analysis, are imperative. These practices make sure that the data collected is robust and defensible, preventing any discrepancies that could compromise the trial’s validity.
Regulatory bodies scrutinize the data management procedures of a clinical trial in detail; thus, rigorous data practices are essential. The integrity of data not only supports a favorable regulatory review but also reinforces the scientific validity of the study, guaranteeing that the findings are reliable and can be confidently applied to patient care.
The Approval Process for Clinical Trials
Securing approval for a clinical trial is critical in developing new medical interventions. This process involves several meticulously planned steps, adherence to regulatory standards, and strategic foresight. Understanding these components can significantly enhance the efficiency and success rate of trial approvals.

Steps Involved in Getting a Clinical Trial Approved
The approval process for clinical trials is structured and requires thorough preparation and strategic alignment with regulatory requirements:
- Preparation of Protocol and Documentation: This initial step involves drafting a detailed clinical trial protocol, which includes study objectives, design, methodology, statistical considerations, and compliance measures. The documentation must also cover participant consent forms, investigator information, and institutional review board (IRB) approvals.
- Submission to Regulatory Authorities: Once the documentation is prepared, it must be submitted to relevant regulatory bodies, such as the FDA in the United States or the EMA in Europe. This submission includes an application that details the trial’s intent, scope, and safety measures.
- Regulatory Review and Responses: After submission, the trial undergoes a review process where regulatory authorities evaluate the proposed plans and safety protocols. During this phase, the sponsor may receive questions or requests for additional information from the regulators.
- Approval and Registration: If the regulatory body is satisfied with the trial’s setup and compliance with safety standards, drug approval is granted. The trial is then registered in a public database, making its details available for public record.
Common Pitfalls and How to Avoid Them
Several common pitfalls can delay or derail the approval of clinical trials. Being aware of these can help address potential issues proactively.
- Insufficient Data on Drug Safety: One of the most significant reasons for the delay or rejection of trial approvals is the inadequate safety data on the investigational drug. To avoid this, ensure thorough preclinical testing and robust safety monitoring plans.
- Inadequate Trial Design: Poorly designed trials that lack clear objectives or valid scientific methodologies can lead to regulatory pushback. Engaging with experts in trial design and conducting feasibility studies beforehand can mitigate this risk.
- Regulatory Misalignment: Misunderstanding or not adhering to the specific regulatory requirements can cause significant setbacks. Continuous engagement with regulatory consultants or regulatory bodies during the trial design phase can ensure alignment with all legal and procedural guidelines.Lack of Patient Recruitment: Difficulty in recruiting a sufficient number of participants can delay the trial and impact data validity. To avoid this, develop a robust recruitment strategy, including patient engagement initiatives, outreach programs, and collaboration with patient advocacy groups.
Importance of a Regulatory Strategy in Clinical Trial Design
Integrating a regulatory strategy into the design of a clinical trial is not just beneficial; it’s essential.
- Align with Regulatory Expectations: Understanding and incorporating regulatory requirements into the trial design from the outset can streamline the approval process and ensure compliance.
- Facilitate Adaptive Designs: Adaptive trial designs that consider potential changes based on interim data analysis can be more efficient and are often looked upon favorably by regulators.
- Enhance Communication with Regulators: Establishing a proactive communication plan with regulatory bodies can help in addressing potential issues early in the trial process, thus avoiding delays and misunderstandings.
- Ensure Ethical Conduct: A well-defined regulatory strategy helps make sure that the trial is conducted ethically, with the best interests of the participants in mind. This includes comprehensive informed consent processes, maintaining participant confidentiality, and carefully weighing the risks and benefits to uphold ethical standards.
Securing Success in Clinical Trials
Mastering the complexities of regulatory affairs is crucial for the success of clinical trials. By adhering to established guidelines and effectively managing the approval process, researchers can greatly increase the chances of favorable outcomes.
Successful clinical trials result from careful planning, vigilant monitoring, and adaptive strategies, paving the way for medical advancements that profoundly impact patient care. If you commit to these principles, you pave the path toward groundbreaking achievements in healthcare research.
[RC4]I also think that a big one here is lack of patient recruitment.
[RC5]Another big one here & I believe they came out with a new guidance not long ago, is regulatory strategy to help ensure that the trial is conducted ethically and with the best interests of the participants of the study (i.e., informed consent forms, participant confidentiality and the risks/benefits of ethical considerations).
