Bringing a new drug to market is a long and highly regulated process designed to ensure safety, efficacy, and quality. The pharmaceutical drug development process moves through multiple stages—beginning with drug discovery, advancing through preclinical and clinical research, and ultimately undergoing rigorous regulatory approval before reaching patients.
Each phase plays a crucial role in evaluating potential treatments, refining formulations, and meeting the stringent standards set by agencies like the FDA and the European Medicines Agency.

Drug Discovery: Identifying a Promising Compound
The pharmaceutical drug development process begins with drug discovery, where researchers search for new chemical compounds that have the potential to become therapeutic agents. This stage is critical—it sets the foundation for everything that follows.
Scientists spend years studying biological processes, identifying potential drug targets, and testing thousands of small-molecule drugs and biologics, hoping to find just a handful of promising compounds that could move forward in the drug development process.
The Role of Basic Research in Understanding Disease Processes
Before scientists can develop a new drug, they first need to understand the disease process at a molecular level. This is where basic research comes in. Researchers—often working in both pharmaceutical companies and academic institutions—study biological processes to determine how diseases progress and what might be done to stop or slow them. They look at existing treatments and search for gaps where innovation is needed.
Finding the Right Molecular Target
Once a disease pathway is understood, the next step is target identification—pinpointing a target molecule that plays a key role in the disease. This could be a protein, enzyme, or receptor that contributes to the condition. The goal is to find a target that, if modified by a drug candidate, could alter the disease’s course.
At this stage, researchers rely on preclinical research and cutting-edge technologies like computational modeling to predict which targets hold the most promise.
Assay Development: Screening for the Most Effective Compounds
With a target identified, researchers turn to assay development, a process that allows them to test thousands of chemical compounds for potential effectiveness. Using automated screening systems, scientists evaluate how different small-molecule drugs or biologics interact with the target. This step is essential for narrowing down the list of potential treatments.
Only a few compounds show the kind of strong, selective action needed to move forward in the drug development process.
Selecting Lead Compounds for Further Investigation
From the initial screening, researchers identify a shortlist of lead compounds—the most promising drug candidates that demonstrate effectiveness against the target while maintaining a good safety profile. These compounds undergo further testing to refine their properties and optimize them for success in later preclinical and clinical research.
The drug discovery and development phase is time-consuming and highly selective. Many drug candidates never make it past this stage, but those that do move forward into the preclinical research phase, where scientists further evaluate their safety and effectiveness before entering human clinical trials.
This rigorous approach ensures that only the most viable drugs advance through the drug development phases, bringing hope for new, effective treatments.

Preclinical Research: Evaluating Safety and Effectiveness
Once a drug candidate has been identified through the drug discovery process, it moves into the preclinical research phase—a crucial step in determining whether the compound is safe and effective enough for human clinical trials.
At this stage, scientists conduct rigorous preclinical trials using animal models and laboratory testing to assess how the drug interacts with biological processes and whether it has the potential to treat the particular disease it was designed for.
Testing Chemical Compounds and Biological Processes
The transition from the discovery process to preclinical research involves extensive testing on both chemical compounds and biologics. Researchers study how the drug candidate behaves in living systems, examining factors like absorption, distribution, metabolism, and excretion. They also assess potential toxicity to determine if the drug poses risks that could prevent it from advancing to human trials.
The Role of Animal Models in Drug Development
Before a new drug can be tested in humans, regulatory agencies such as the FDA and the European Medicines Agency require it to undergo preclinical studies using animal models. These models help scientists predict how a drug candidate will interact within a living system, providing critical data on safety, dosing, and potential side effects. While animal studies cannot perfectly replicate human responses, they serve as an essential step in filtering out compounds that could be too toxic or ineffective before progressing further in the drug development process.
Determining If a Drug Candidate Moves to Human Trials
The data gathered during preclinical research plays a key role in deciding whether a drug candidate is ready for clinical testing. Researchers analyze results from preclinical and clinical research comparisons to assess the drug’s potential benefits and risks.
If the findings are promising, the drug’s developers prepare to submit an Investigational Drug Application (IND) to regulatory agencies, requesting permission to proceed with human clinical trials.
Formulating a Drug for Clinical Testing
An important part of preclinical research is developing a pharmaceutical formulation that can be safely administered to patients. Scientists determine the optimal dosage, delivery method (such as oral, intravenous, or topical), and stability of the drug. This formulation must be suitable for use in clinical development, ensuring that researchers can accurately test its effects during clinical trials.
By the end of the preclinical research phase, only a few compounds from the original drug discovery and development efforts make it through to starting clinical trials. The rigorous testing conducted in this phase helps filter out unsafe or ineffective drugs, ensuring that only the most viable treatments move forward into human clinical trials, where their safety and efficacy can be further evaluated.
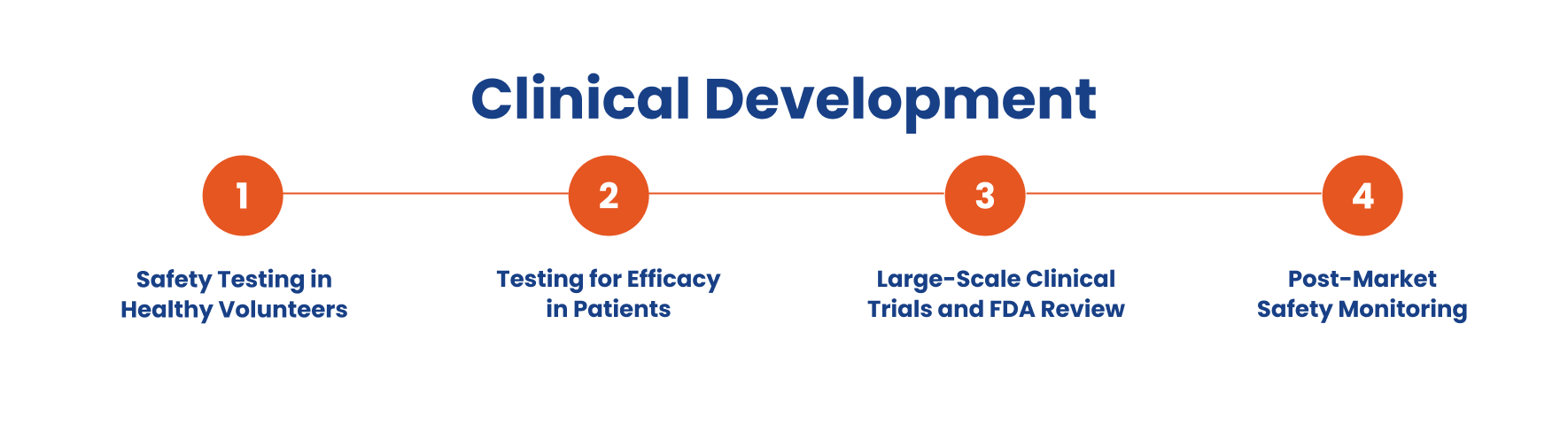
Clinical Development: Human Trials and Regulatory Review
Once a drug candidate successfully passes preclinical research, it moves into clinical development, where it is tested in humans to evaluate its safety, efficacy, and overall benefit-risk profile. This multi-phase process is closely regulated by agencies like the FDA and the European Medicines Agency to ensure that only safe and effective treatments reach patients.
- Phase I: Safety Testing in Healthy Volunteers – The clinical development process begins with Phase I trials, where a new drug is tested in a small group of healthy volunteers (20–100) to assess drug safety, metabolism, and optimal dosage. Researchers monitor for side effects and determine how the body processes the drug. If it is well tolerated, it moves to the next phase.
- Phase II: Testing for Efficacy in Patients – In Phase II trials, the drug is given to patients with a particular disease (100–300) to evaluate medication effectiveness and compare it to existing treatments. Researchers analyze how the drug interacts with biological processes while continuing to monitor safety.
- Phase III: Large-Scale Clinical Trials and FDA Review – If effective, the drug enters Phase III clinical trials, where thousands of participants help confirm its safety and efficacy. These trials compare the new drug development against approved drugs. Successful results lead to a drug application submission to regulatory agencies like the FDA or European Medicines Agency for regulatory approval.
- Phase IV: Post-Market Safety Monitoring – After market approval, post-market safety monitoring (Phase IV) ensures long-term safety and effectiveness in a broader population. Drug developers track rare side effects, conduct additional clinical trials, and refine treatment protocols to maintain clinical practice standards.
Related Article: Accelerating Drug Development
Regulatory Approval The Path to Market
Before a new drug can reach patients, it must undergo a rigorous regulatory approval process. The FDA requires drug companies to submit a drug application before advancing to clinical trials, ensuring that the treatment meets safety and ethical standards. This application includes preclinical and early clinical data outlining the drug’s potential benefits and risks.
Once a drug successfully completes Phase III clinical trials, pharmaceutical companies submit a New Drug Application (NDA) for small molecule drugs or a Biologics License Application (BLA) for biologic therapies. During the FDA review, regulators assess the drug’s safety, efficacy, and manufacturing quality. This process can take months or even years, depending on the complexity of the treatment.
Beyond the FDA, global regulatory agencies like the European Medicines Agency play a crucial role in drug approval, ensuring that treatments meet international safety and efficacy standards. These agencies often collaborate, allowing pharmaceutical companies to seek approval across multiple regions.
Regulators may classify a drug under standard or priority review, depending on its urgency. Drugs that address unmet medical needs or offer significant advantages over existing treatments may receive priority review, expediting the process to bring life-saving therapies to patients faster.
Bringing New Medications to Patients Safely and Effectively
The pharmaceutical drug development process is a complex, multi-stage journey that ensures only the safest and most effective treatments reach patients. From drug discovery and preclinical research to clinical trials and regulatory approval, each step is designed to evaluate a drug’s safety, efficacy, and overall benefit.
While only a few compounds make it through the rigorous testing phases, those that do have the potential to transform lives and advance modern medicine. As science and technology continue to evolve, the future of drug development holds even greater promise for innovative therapies and life-saving breakthroughs.

