
Maria Wik, MS
Maria Wik, MS, has worked in the biotechnology industry since 2002, focusing on development and commercialization of recombinant proteins, monoclonal antibodies, cell and gene therapies in various Process Development, Manufacturing Science and Technology (MSAT), and Manufacturing roles.
Maria began her career at Amgen, where she developed expertise in process development, process characterization, technology transfer, process validation, and commercial manufacturing working with process scales ranging from bench through large scale 20kL bioreactors. She gained experience working with microbial and mammalian processes, upstream and downstream operations, using single-use and stainless-steel technologies.
From 2016-2019, Maria was a part of the Site Leadership Team at the AstraZeneca Colorado manufacturing site and led the MSAT team as the Director of MSAT. Most recently, prior to joining Syner-G (RMC Pharmaceutical Solutions) in 2021, Maria was the Sr Director of Manufacturing at Avexis/Novartis Gene Therapies. In this role, Maria led a team of 130 manufacturing staff to successful facility start-up and first GMP manufacturing batch within 10 months of site acquisition.
At Syner-G, Maria enjoys working with clients to develop and execute their CMC strategy to bring impactful treatments to patients.
Maria received her M.S. in Chemical Engineering from University of Colorado Boulder, and her B.S. from Hogskolan Dalarna, Sweden.
Maria Wik, MS's Recent Articles
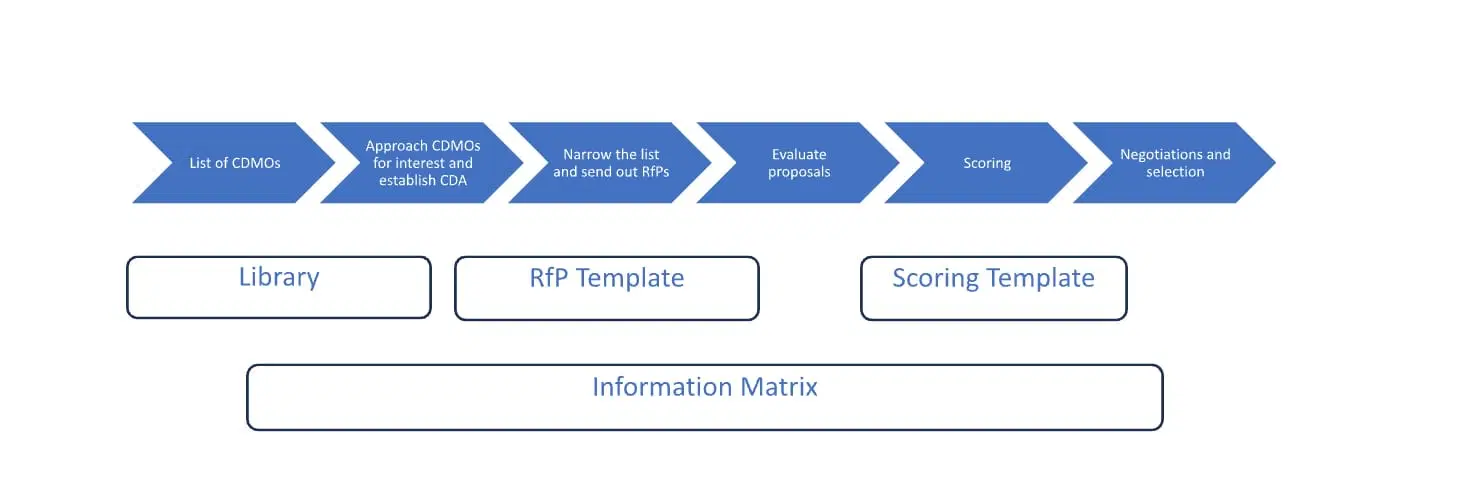
Useful Tools to Facilitate CDMO Selection Process for Biologics
Selecting a Contract Development and Marketing Organization (CDMO) is not a new topic, and much has been written and learned since CDMO’s first started to become an option in the late 1990s. If the decision is to outsource, selecting a…
