The simple answer is you save money, time and get an exciting new drug to the clinic for patients.
What is Regulatory CMC and why do I need to pay attention to this during development? Regulatory CMC is at the epicenter of clinical development (see Figure 1), and with an experienced leader, connecting and navigating the various areas (or silos) in clinical development is essential.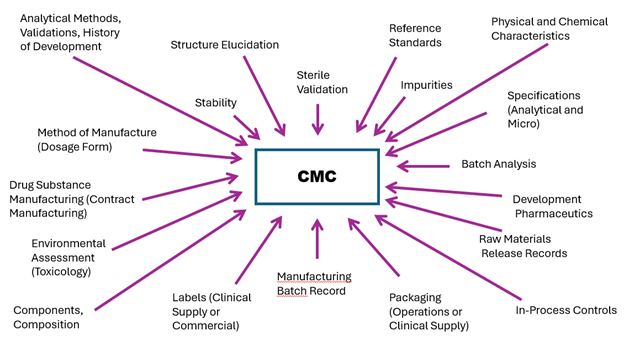
Figure 1. Components of Chemistry, Manufacturing and Controls (CMC)
What are some of the pitfalls an experienced Regulatory CMC Consultant can help you avoid?
- Spending money on laboratory studies may not be needed for early phase studies and may need to be repeated later in development. For example, extractables and leachable studies are not needed until a final container closure system is chosen.
- Not anticipating supply chain disruptions/changes and not having a regulatory strategy to manage. For instance, moving to a new manufacturer and not planning for regulatory comparability if needed in a timely manner
- Inability to release drug product. For example, relying on inadequate analytical methods that required more method development and therefore quality issues such as out of specification events appear
- Avoiding appropriate investing in early development on key CMC areas to streamline going into Phase 2 and Phase 3. For instance, limited number of batches manufactured using an early process and consistency has not been shown prior to moving to a new process
- Unanticipated Clinical Hold based on CMC issues
Experience Matters and a Good Team Matters! Syner-G has the Regulatory CMC Consultants with the experience you NEED to avoid the pitfalls that can negatively impact your development program. Our experts can not only help get your IND/IMPDs cleared, but we can also do it while saving you time and money.


