August 25, 2016 | Kim Nice, PhD, Associate Director, Medical Writing and Submissions Management | Medical Writing Services
In previous posts, you were introduced to the role of regulatory medical writing and heard about the day-to-day activities of a regulatory medical writer. In this post, I will tell you about the skills you need to be good at the job.
| In my role at IMPACT, I spend a good deal of my time mentoring our staff on regulatory medical writing.
I am often asked “What makes a good regulatory medical writer?” My initial reaction was to say “being a good scientist”, but when I gave it a little more thought, I realized there are many aspects to mastering this job that include:
Read on for more specifics about each of these required skill sets… |
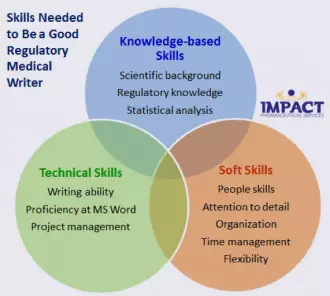 |
The following knowledge-based skills are fundamental for being a good regulatory medical writer:
- Having a strong scientific background: Being a well-trained scientist is fundamental. You will utilize the skills you learned at the bench, such as understanding study design and scientific methods, the ability to analyze data, and how to clearly communicate results.
- Regulatory knowledge: Understanding the “big picture” of drug development and awareness of drug development regulations is important. Fortunately, this type of information can usually be learned on the job, since most folks coming from academia will have had little to no exposure to this.
- Understanding statistical analysis: If you aren’t a stats whiz yet, you will be! You will be interpreting data from clinical studies, which use a variety of statistical methods. Having a basic familiarity of statistical tests and methods is a plus.
These technical skills are essential to the job of a regulatory medical writer:
- Writing ability: This is not creative writing! As the saying goes: “just the facts, ma’am.”
Good writing ability for a regulatory medical writer means that you can clearly and succinctly communicate the content, provide a consistent and logical structure, and use correct grammar. (Hopefully you have been developing these skills through preparing posters and writing journal articles.)
- Proficient use of Microsoft Word: You may think you know how to use Word pretty well (I did before I started this job), but you are probably wrong!
As a regulatory medical writer, you will need to become a savvy Word user. This is particularly important when working at a CRO (contract research organization), where you are often using different templates/styles for different clients.
Correct formatting is not only visually important, but is technically important for a document to be “submission ready” and published electronically (see posts from IMPACT’s BJ Witkin on electronic publishing).
- Project management: Good project management skills are essential to being able to plan and organize the many steps needed to produce the finished product, as well as manage the budget. (I bet you have had lots of practice with this by managing your lab work.)
This is the category that surprises people most often. A good medical writer needs to have good people skills, patience, and a high (some might say “obsessive”) level of attention to detail; be able to organize, prioritize, and multi-task; and on top of all that, be flexible.
- People skills and patience: Despite the visual image that you may have of a writer sitting at a computer all day, a good chunk of time is spent interacting with our team/colleagues and/or our clients.
People skills and patience are essential as medical writers are often in the role of leader, advisor, negotiator, and mediator.
Since we work on teams that are made up of experts from various fields and it is our job to drive consensus, you can imagine how challenging it can be at times to manage these different (and sometimes strong) personalities. (I wish I had paid better attention in that psychology course in college!)
So, if you are considered the “diplomat” in your family or circle of friends and the kind of person that doesn’t get rattled easily, then this job may be for you.
- Attention to detail: When producing a high-quality regulatory document, not only does the content need to be exacting, but the little details need to be correct, too.
As medical writers, it is our job to make sure that the text is written consistently, styles are adhered to, and the formatting is correct, so attention to detail is a must.
The more you do this job, the more you will find yourself noticing every typo and grammatical mistake on a restaurant menu, advertisement, website, etc (this makes you a good QC’er, too!). [Don’t remember what QC is? Find out here!]
- Organize, prioritize, and multi-task: As medical writers, we not only write, but we also organize all the necessary tasks involved in generating a finished product (eg, QC, review, publishing), as well as communicate with our clients – often on a daily basis.
Keep in mind that we may be doing this for multiple projects at the same time. Therefore, being able to manage your time, prioritize your work load (daily, and sometime hourly!), and multi-task is a must in order to be a successful medical writer.
Medical writers as a group are sometimes accused of being the type of people who organize their sock drawer and/or spice rack, generate a to-do list and a checklist for just about everything, and get excited by the prospect of arranging all the junk in the garage (okay, maybe this last one is just me).
If this sounds like you, then you may enjoy a career in medical writing.
- Be flexible: Regardless of where you work, but especially if you are at a CRO (like IMPACT), you must, above all, be flexible!
Remember that a regulatory medical writer at a CRO is a service provider, and when the client’s needs and timelines shift, we have to shift too. This fluidity is what keeps me on my toes!
If the thought of deviating from the plan makes you break out in hives, then you may want to consider a different career path (or at least avoid working for a CRO).
Is Being a Regulatory Medical Writer Right for YOU?
| Hopefully I have dispelled the image that medical writers type on a computer all day, never interacting with others.
But as you can see from this post, a good regulatory medical writer draws on a wide array of skills that go beyond just being able to articulate medical information in a clear and concise manner. With roles like scientist, project manager, team player, leader, and mediator, and tasks such as writing, interpreting data, organizing, juggling, and re-prioritizing, it’s no wonder our days fly by! |
|
If you want to know more about being a regulatory medical writer, whether at IMPACT or in general, please don’t hesitate to contact us. And if you think you’d like to work with us at IMPACT, visit our careers page.

Category: Medical Writing Services,
Keywords: medical writing, regulatory medical writing, medical writer, regulatory medical writer
Other Posts You Might Like: